
Sia

Question
Chemistry
Posted over 1 year ago
1. Answer the following miscellaneous questions:
(4 pts)
b) Draw the most stable conformational structure for 2methylpentane looking down at 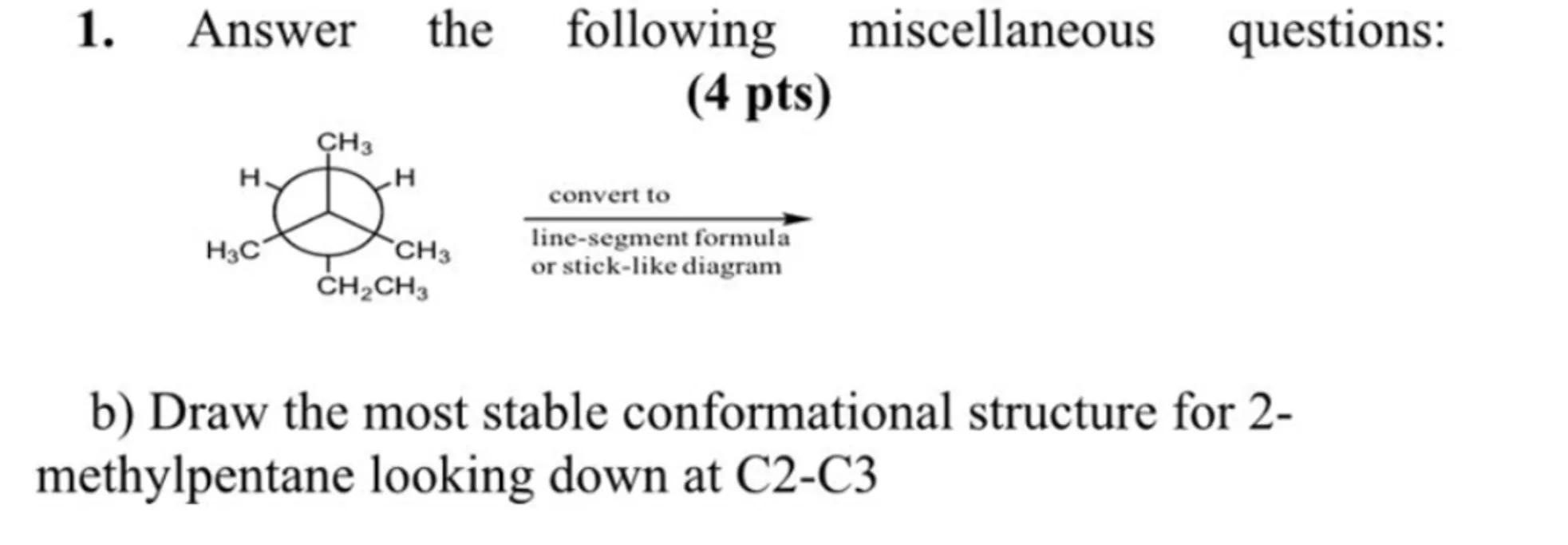

Answer from Sia
Posted over 1 year ago
Solution
Question 1: Convert the provided chemical structure to a line-segment formula or stick-like diagram.1
Identify the structure: Examine the provided chemical structure to understand the arrangement of atoms and bonds
2
Simplify the structure: Convert the detailed chemical structure into a line-segment formula by representing carbon atoms as vertices and bonds as lines. Hydrogen atoms attached to carbons are usually omitted for simplicity
3
Draw the line-segment formula: Sketch the simplified structure, ensuring that the correct number of bonds and the overall shape of the molecule are maintained
Answer
[Insert final line-segment formula here]
Key Concept
Line-segment formulas simplify the representation of organic molecules by focusing on the carbon backbone and omitting hydrogen atoms attached to carbons.
Explanation
This method of drawing organic molecules helps to quickly convey the structure without unnecessary details, making it easier to understand and analyze.
1
Identify the molecule: Recognize that 2-methylpentane is an alkane with a methyl group attached to the second carbon of a pentane chain
2
Determine the conformation: Understand that the most stable conformation for alkanes is typically the staggered conformation, where atoms are positioned to minimize repulsion
3
Draw the Newman projection: Looking down the C2-C3 bond, draw the Newman projection with the front carbon (C2) and the back carbon (C3). Arrange the substituents (hydrogen and methyl groups) to achieve the staggered conformation
Answer
[Insert final Newman projection here]
Key Concept
The most stable conformation of alkanes is the staggered conformation, which minimizes electron repulsion between adjacent atoms.
Explanation
In the staggered conformation, the atoms or groups attached to the carbons are positioned as far apart as possible, reducing torsional strain and resulting in a more stable structure.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question