
Sia
1) Consider the following diagram for a chemical system containing three substances represented by , and :
a) What feature of the graph indicates that the system reaches equilibrium? (1 marks)
b) Write a balanced equation for the equilibrium reaction. (2 marks)
c) Calculate the at equilibrium. ( 3 marks)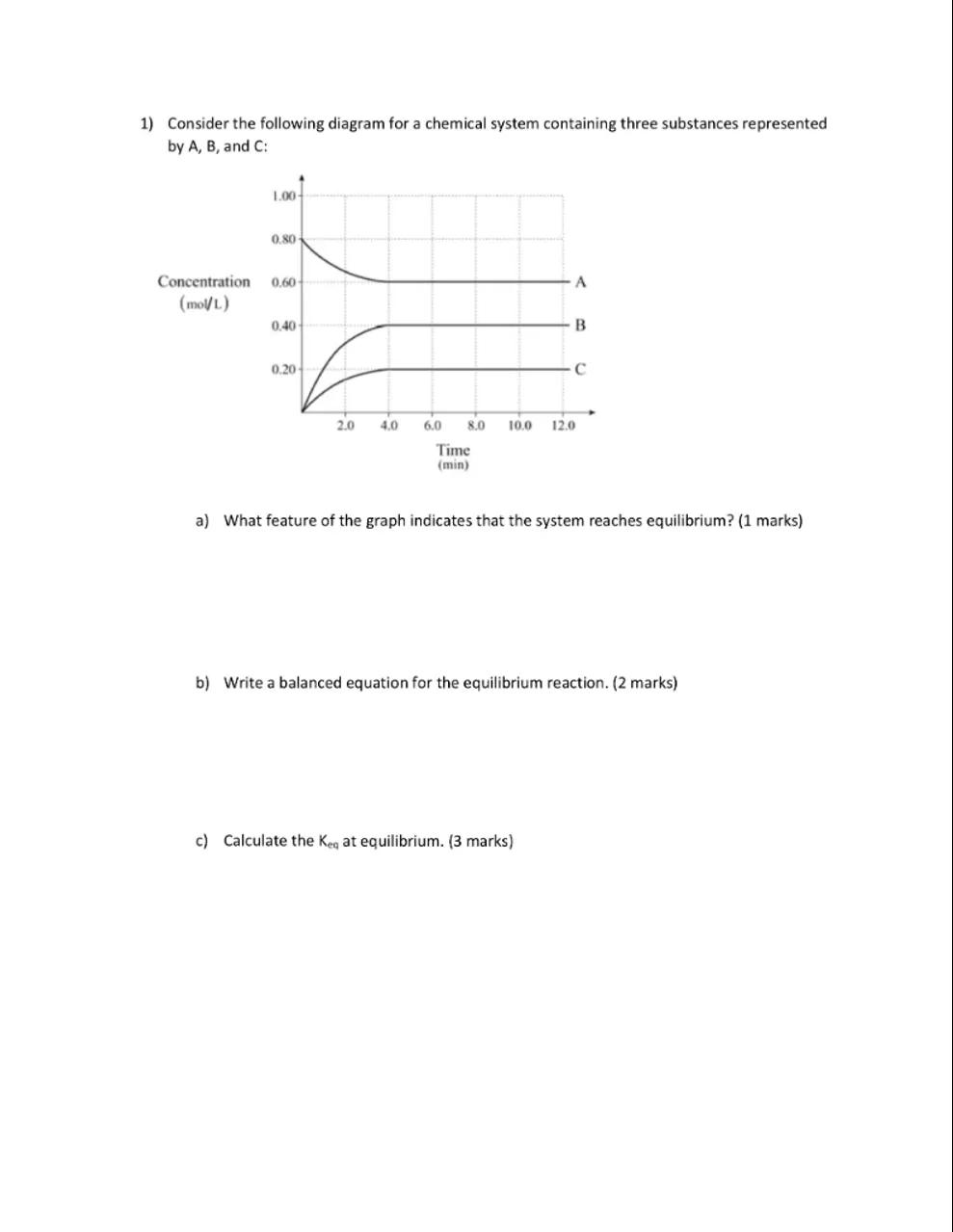

Answer from Sia
Posted almost 2 years ago
Solution
1
Identify the equilibrium point: The feature of the graph that indicates the system reaches equilibrium is the point where the concentrations of all substances become constant over time
2
Determine the balanced equation: To write a balanced equation for the equilibrium reaction, we need to consider the behavior of substances A, B, and C. Since A decreases and B and C increase, a possible reaction could be . However, without additional information about the stoichiometry of the reaction, we can only assume the simplest form where the coefficients of all substances are 1
3
Calculate the equilibrium constant $K_{\text{eq}}$: To calculate , we need the equilibrium concentrations of A, B, and C. Assuming the graph provides these values at the plateau, we use the expression . The exact values need to be read from the graph. If, for example, at equilibrium , , and , then
1 Answer
The graph indicates that the system reaches equilibrium when the concentrations of all substances become constant over time.
Key Concept
Equilibrium is reached when the concentrations of reactants and products remain constant over time.
Explanation
The graph shows that the concentrations of A, B, and C level off, indicating no further change and thus equilibrium.
2 Answer
The balanced equation for the equilibrium reaction is .
Key Concept
A balanced chemical equation represents the conservation of mass in a reaction.
Explanation
Without additional information, the simplest whole number ratio of reactants to products is assumed to be 1:1:1.
3 Answer
Assuming the equilibrium concentrations are , , and , the equilibrium constant would be 0.90.
Key Concept
The equilibrium constant quantifies the ratio of product concentrations to reactant concentrations at equilibrium.
Explanation
The value of is calculated using the equilibrium concentrations of the reactants and products, which must be determined from the graph.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question
