
Sia
1. a. (i) Identify the alpha-hydrogens in compounds A and B shown below. Provide approximate values for these hydrogens. Account for your answers using resonance structures.
A
B
[8 marks]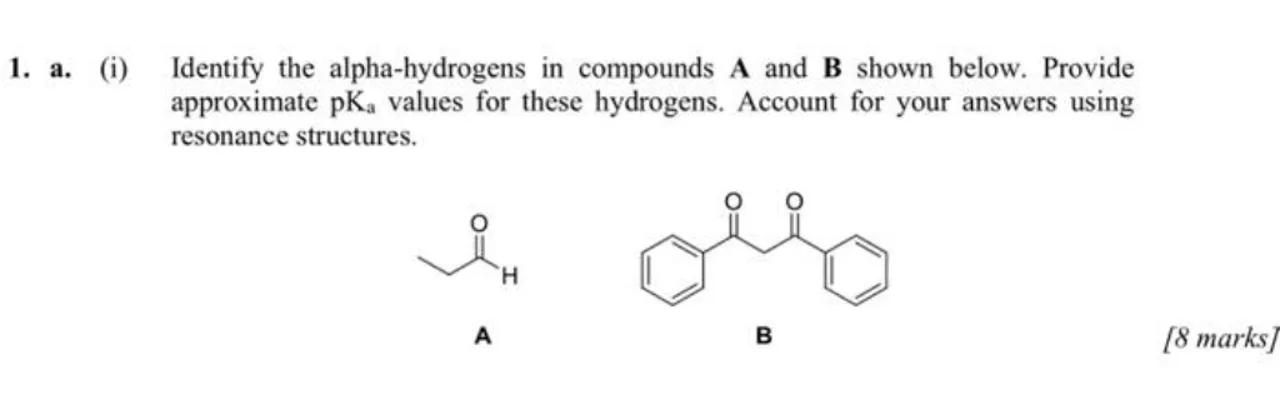

Answer from Sia
Posted almost 2 years ago
Solution
1
Identify alpha-hydrogens in compound A: Alpha-hydrogens are those attached to the carbon adjacent to a carbonyl group. In compound A, there is one alpha carbon with one hydrogen attached to it
2
Approximate $\mathrm{pK}_\mathrm{a}$ value for alpha-hydrogens in compound A: The of an alpha-hydrogen in a simple carbonyl compound like compound A is typically around 20
3
Identify alpha-hydrogens in compound B: Compound B has two aromatic rings connected by a carbonyl group. The alpha-hydrogens are those on the carbons adjacent to the carbonyl group. There may be multiple alpha-hydrogens due to the presence of multiple alpha carbons
4
Approximate $\mathrm{pK}_\mathrm{a}$ value for alpha-hydrogens in compound B: The values for alpha-hydrogens in compound B are influenced by the resonance stabilization provided by the aromatic rings. These values are typically higher than those of simple carbonyl compounds, often in the range of 20-25
5
Account for answers using resonance structures: Resonance structures can delocalize the negative charge after deprotonation of the alpha-hydrogen, stabilizing the resulting enolate ion. This stabilization is greater in compound B due to the aromatic rings, which can contribute to the resonance stabilization
Answer
Compound A has one alpha-hydrogen with a of approximately 20. Compound B has multiple alpha-hydrogens with values typically in the range of 20-25, influenced by resonance stabilization from the aromatic rings.
Key Concept
Alpha-hydrogens are located on the carbon adjacent to a carbonyl group, and their values are influenced by the ability of the molecule to stabilize the negative charge through resonance.
Explanation
The values of alpha-hydrogens are approximated based on the stability of the conjugate base formed after deprotonation. Resonance structures that delocalize the negative charge increase this stability, leading to higher values.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question