
Sia

Question
Chemistry
Posted almost 2 years ago
12.11 Using values for and at 298 kelvins, estimate the standard boiling point of mercury, and for . Determine the vapor pressure of mercury at .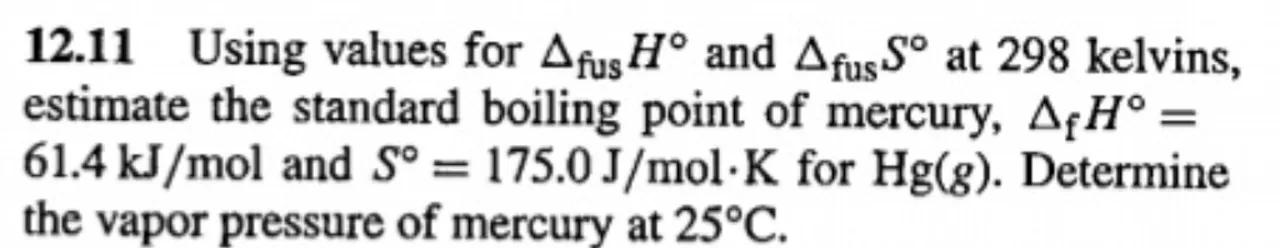

Answer from Sia
Posted almost 2 years ago
Solution
1
Use the Gibbs free energy equation for phase transition: To estimate the standard boiling point of mercury, we can use the Gibbs free energy equation . At the boiling point, for the phase transition is zero
2
Solve for the boiling point: Setting for the boiling point, we get . We can solve for the boiling temperature by rearranging the equation:
3
Calculate the boiling point: Plugging in the values,
4
Determine the vapor pressure at $25^{\circ}C$: To find the vapor pressure at (or ), we can use the Clausius-Clapeyron equation, which relates the vapor pressure at two temperatures. However, we need the heat of vaporization and the vapor pressure at another known temperature, which are not provided. Without this information, we cannot calculate the vapor pressure at
Answer
The standard boiling point of mercury is approximately 350.857 K. The vapor pressure at cannot be determined with the given information.
Key Concept
The boiling point of a substance can be estimated using the Gibbs free energy equation for phase transitions.
Explanation
At the boiling point, the Gibbs free energy change for the phase transition from liquid to gas is zero, allowing us to solve for the boiling temperature using the enthalpy and entropy of fusion.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question