
Sia
2. (30 pts) By using the same alkene, how do you synthesize the following alcohols? Give the alkene and show the mechanisms for each product formation.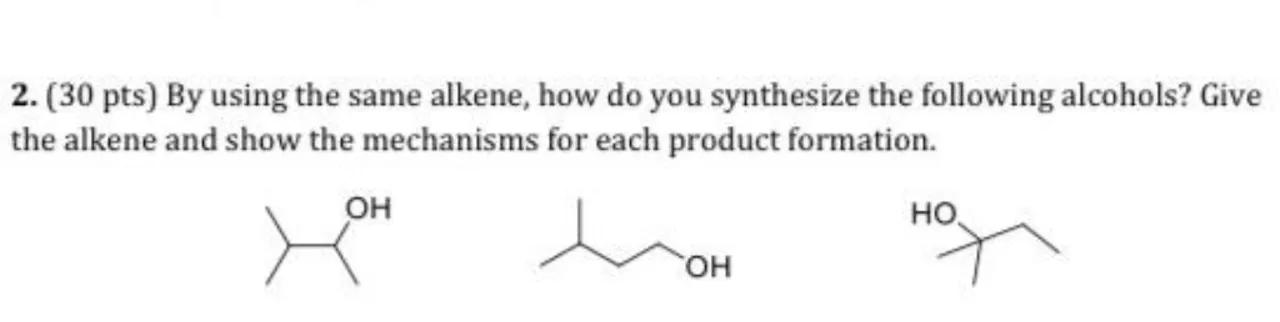

Answer from Sia
Posted almost 2 years ago
Solution
1
Identify the starting alkene: To synthesize the given alcohols from the same alkene, we must first identify a suitable alkene that can be transformed into all three alcohols. The molecular formula for the alcohols is , which suggests that the alkene should have four carbon atoms and be able to form alcohols at different positions. The suitable starting alkene is but-2-ene, which has the formula
2
Synthesize the first alcohol: The first alcohol is a tertiary alcohol, which can be synthesized from but-2-ene using acid-catalyzed hydration. This reaction follows Markovnikov's rule, where the group attaches to the more substituted carbon atom. The mechanism involves the formation of a carbocation intermediate at the tertiary carbon, followed by the attack of a water molecule and deprotonation to form the tertiary alcohol
3
Synthesize the second alcohol: The second alcohol is a primary alcohol, which can be synthesized from but-2-ene using hydroboration-oxidation. This anti-Markovnikov reaction involves the addition of borane () to the less substituted carbon of the alkene, followed by oxidation with hydrogen peroxide () to replace the boron atom with a hydroxyl group, yielding the primary alcohol
4
Synthesize the third alcohol: The third alcohol can be synthesized by first converting but-2-ene into a secondary alcohol using acid-catalyzed hydration, similar to the first alcohol, but with a different regioselectivity. This can be achieved by using a different reaction condition or catalyst that favors the formation of the secondary carbocation intermediate. The water molecule then attacks the secondary carbocation, followed by deprotonation to form the secondary alcohol
Answer
The starting alkene for the synthesis of all three alcohols is but-2-ene. The first tertiary alcohol is synthesized via acid-catalyzed hydration following Markovnikov's rule. The second primary alcohol is synthesized via hydroboration-oxidation following anti-Markovnikov's rule. The third secondary alcohol is synthesized via acid-catalyzed hydration with different regioselectivity.
Key Concept
Synthesis of alcohols from alkenes
Explanation
Alcohols can be synthesized from alkenes using different reactions such as acid-catalyzed hydration and hydroboration-oxidation, which follow Markovnikov's and anti-Markovnikov's rules, respectively, to determine the position of the hydroxyl group.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question
