
Sia
2. A new element with atomic number 116 was discovered in 2000 . In 2012 it was named livermorium, Lv. Although is radioactive and short-lived, its chemical properties and reactivity should follow periodic trends.
a. Write the electron configuration for the valence electrons of in the ground state.
b. According to periodic properties, what would be the most likely formula for the product obtained when Lv reacts with ?
c. The first ionization energy of polonium, Po, is . Is the first ionization energy of expected to be greater than, less than, or equal to that of Po? Justify your answer in terms of Coulomb's law.
d. Shown below is a hypothetical mass spectrum for a sample of containing 10 atoms.
Using the information in the graph, determine the average atomic mass of in the sample to four significant figures.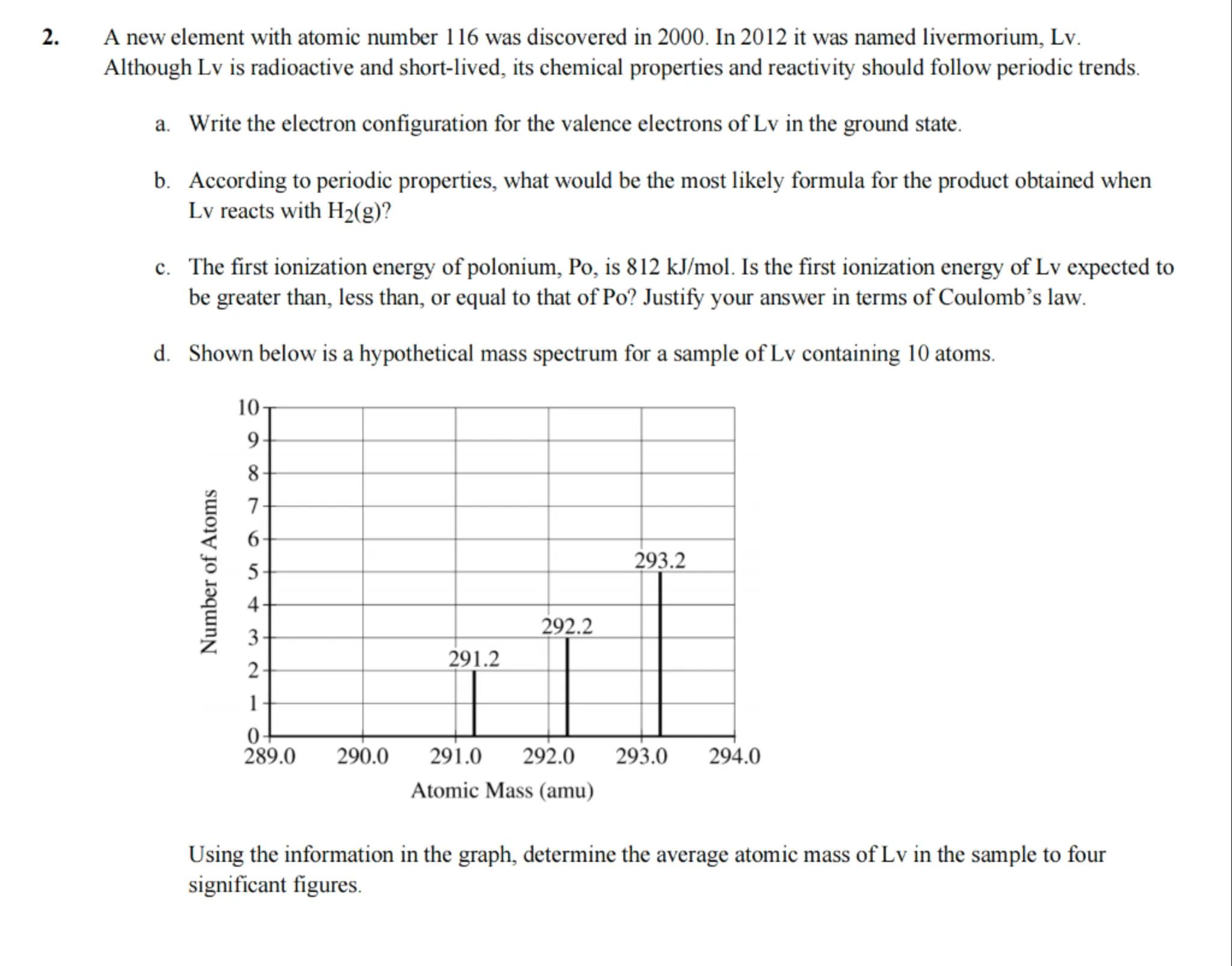

Answer from Sia
Posted over 1 year ago
Sure, let's address each part of the student's question one by one.
Part a: Electron Configuration for Valence Electrons of Lv
A
Key Concept
Electron Configuration
Explanation
The valence electron configuration for Livermorium (Lv) in the ground state is .
Part b: Most Likely Formula for the Product with
B
Key Concept
Periodic Trends and Reactivity
Explanation
Based on periodic trends, Livermorium (Lv) is in the same group as oxygen and sulfur, which form and respectively. Therefore, the most likely formula for the product when Lv reacts with is .
Part c: First Ionization Energy of Lv Compared to Po
C
Key Concept
Coulomb's Law and Ionization Energy
Explanation
According to Coulomb's law, the ionization energy depends on the effective nuclear charge and the distance of the valence electrons from the nucleus. Since Livermorium (Lv) is below Polonium (Po) in the periodic table, it has more electron shells, which increases the distance of the valence electrons from the nucleus and decreases the effective nuclear charge. Therefore, the first ionization energy of Lv is expected to be less than that of Po.
Part d: Average Atomic Mass of Lv
D
Key Concept
Average Atomic Mass Calculation
Explanation
To calculate the average atomic mass, use the formula: . For the given data: . The total number of atoms is . Therefore, the average atomic mass is amu.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question
