
Sia
3. a. The reaction sequence shown in Figure 4 demonstrates the synthesis of a disubstituted benzene derivative from compound through two Electrophilic Aromatic Substitution reactions.
(i) Draw the chemical structure of compound .
[2 marks]
(ii) Draw the mechanism for the transformation of compound to intermediate using curly arrows.
[6 marks]
(iii) Draw the chemical structure of compound .
[2 marks]
(iv) Draw the mechanism for the transformation of intermediate to compound using curly arrows.
[6 marks]
(v) Account for the regiochemical outcome observed in compound .
[4 marks]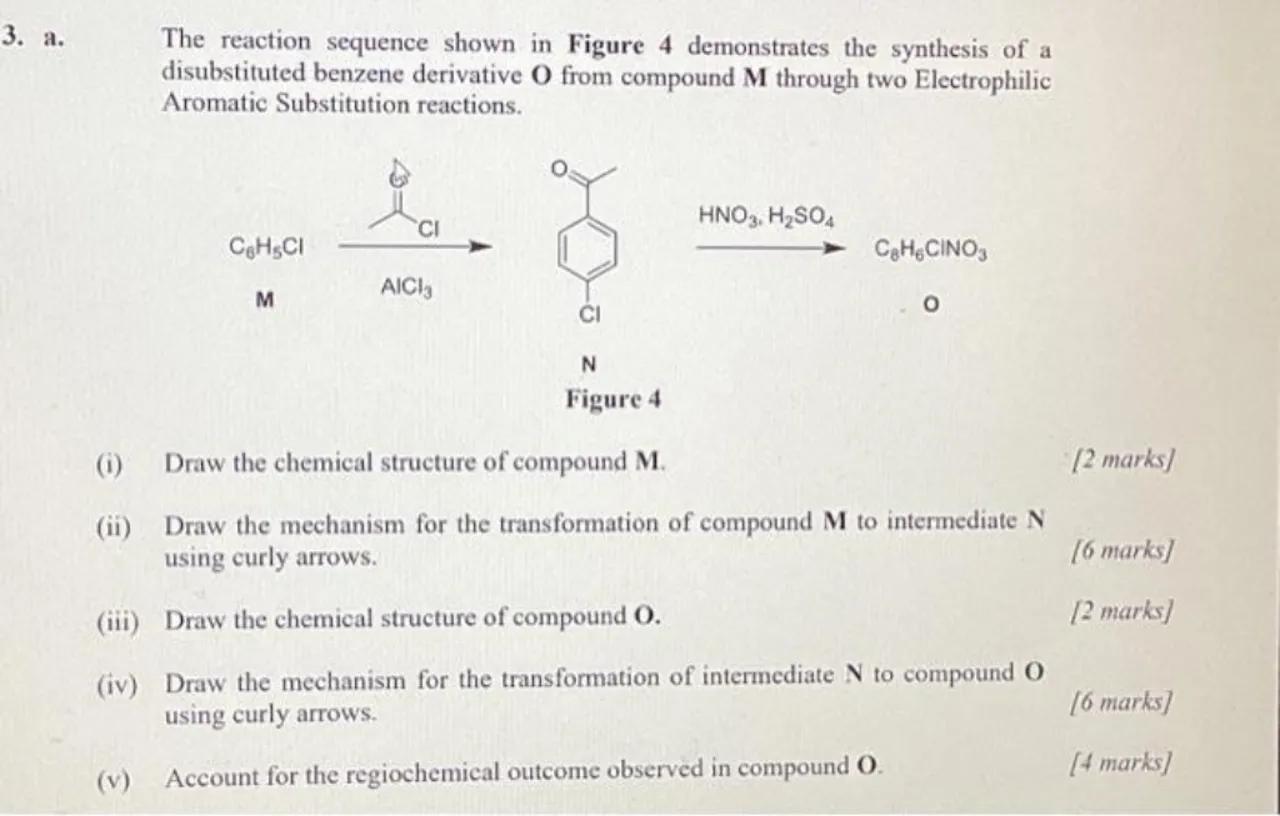

Answer from Sia
Posted almost 2 years ago
Solution
1
Draw the chemical structure of compound $M$: Compound is given as , which is chlorobenzene. The structure is a benzene ring with a chlorine atom attached to one of the carbon atoms
2
Draw the mechanism for the transformation of compound $M$ to intermediate $N$ using curly arrows: The reaction of chlorobenzene () with is an example of an electrophilic aromatic substitution (EAS) reaction. The acts as a Lewis acid, facilitating the formation of the electrophile . The mechanism involves the following steps:
1. Formation of the electrophile: reacts with to form and .
2. The benzene ring donates a pair of electrons to the , forming a sigma complex (arenium ion).
3. The abstracts a proton from the sigma complex, restoring the aromaticity and forming the product (chlorobenzene)
3
Draw the chemical structure of compound $O$: Compound is given as , which is a benzene ring with a chlorine atom and a nitro group () attached. The structure is a benzene ring with a chlorine atom at one position and a nitro group at another position
4
Draw the mechanism for the transformation of intermediate $N$ to compound $O$ using curly arrows: The reaction of chlorobenzene () with and is another example of an electrophilic aromatic substitution (EAS) reaction. The acts as a catalyst, facilitating the formation of the electrophile . The mechanism involves the following steps:
1. Formation of the electrophile: reacts with to form and .
2. The benzene ring donates a pair of electrons to the , forming a sigma complex (arenium ion).
3. The abstracts a proton from the sigma complex, restoring the aromaticity and forming the product (chloronitrobenzene)
5
Account for the regiochemical outcome observed in compound $O$: The regiochemistry of the nitration of chlorobenzene is influenced by the electron-withdrawing nature of the chlorine substituent. Chlorine is an ortho/para-directing group due to its lone pairs, which can donate electron density through resonance. However, it is also an electron-withdrawing group through induction. The net effect is that nitration occurs preferentially at the ortho and para positions relative to the chlorine substituent. In this case, the product is likely to be a mixture of ortho- and para-chloronitrobenzene, with the para product being more predominant due to steric hindrance at the ortho position
Answer
The chemical structures and mechanisms for the transformations of compounds to and to involve electrophilic aromatic substitution reactions, with the regiochemistry influenced by the substituents on the benzene ring.
Key Concept
Electrophilic Aromatic Substitution (EAS)
Explanation
EAS reactions involve the substitution of a hydrogen atom on an aromatic ring with an electrophile. The regiochemistry of the substitution is influenced by the nature of the substituents already present on the ring.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question