
Sia
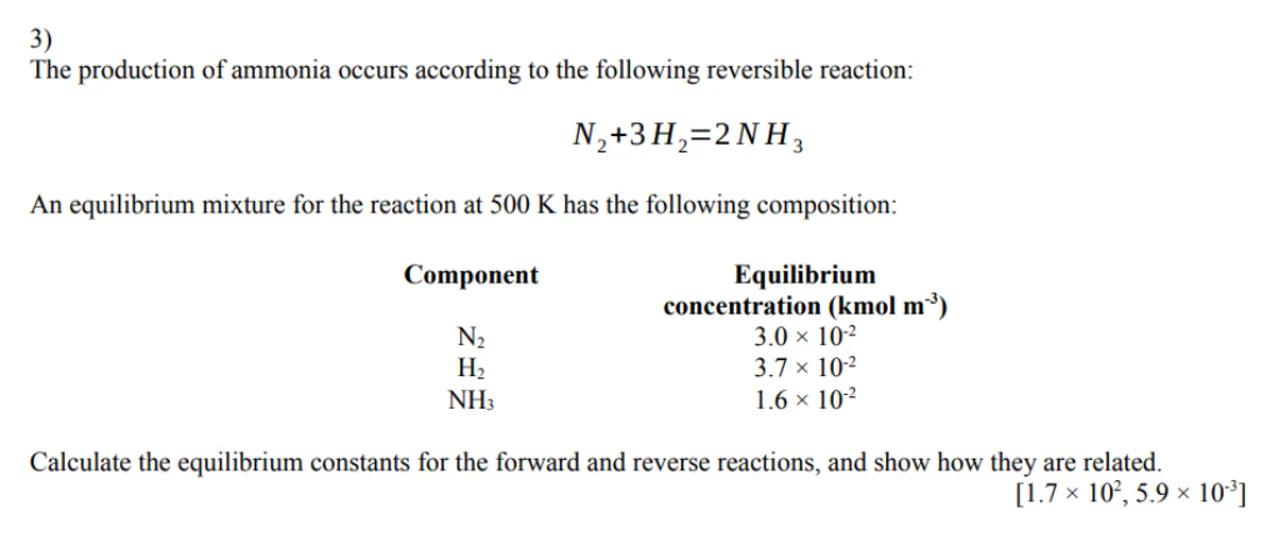
3)
The production of ammonia occurs according to the following reversible reaction:
An equilibrium mixture for the reaction at has the following composition:
\begin{tabular}{|c|c|}
\hline Component & \begin{tabular}{c}
Equilibrium \\
concentration
\end{tabular} \\
\hline & \\
\hline & \\
\hline & \\
\hline
\end{tabular}
Calculate the equilibrium constants for the forward and reverse reactions, and show how they are related.

Answer from Sia
Posted almost 2 years ago
Solution
1
Write the expression for the equilibrium constant (Kc) for the forward reaction: The equilibrium constant for the forward reaction can be expressed in terms of the concentrations of the products and reactants raised to the power of their stoichiometric coefficients. For the reaction , the equilibrium constant is given by
2
Calculate the equilibrium constant for the forward reaction (Kc): Using the given equilibrium concentrations, we can substitute them into the expression for . Thus, . Calculating this gives
3
Write the expression for the equilibrium constant (Kc') for the reverse reaction: The equilibrium constant for the reverse reaction is the inverse of the equilibrium constant for the forward reaction,
4
Calculate the equilibrium constant for the reverse reaction (Kc'): Using the value of from step 2,
Answer
[1.7 × 10^2, 5.9 × 10^-3]
Key Concept
The equilibrium constant for a reaction is a ratio of the concentrations of products to reactants, each raised to the power of their stoichiometric coefficients. For the reverse reaction, the equilibrium constant is the inverse of the forward reaction's equilibrium constant.
Explanation
The calculated equilibrium constants for the forward and reverse reactions show the relationship between them, where the product of the two constants is equal to 1, demonstrating the reciprocal nature of these constants in a reversible chemical reaction.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question
