
Sia
4. (30 pts) Propose a synthesis route for the following conversion. Give the appropriate reagents needed (it may include more than one step).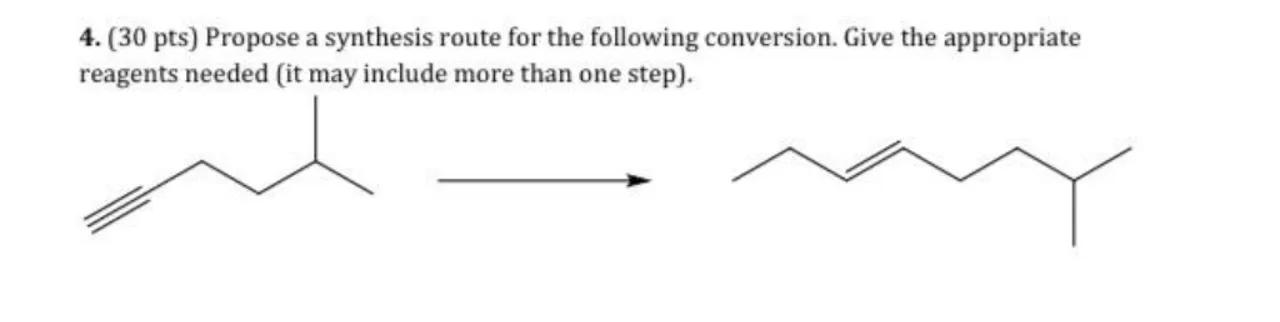

Answer from Sia
Posted almost 2 years ago
Solution
1
Identify the starting material and product: The starting material has a triple bond, which suggests it is an alkyne, and the product has multiple double bonds, indicating an alkene. The presence of a methyl group indicates that the starting material is a substituted alkyne
2
Propose a synthesis route: To convert an alkyne to an alkene with multiple double bonds, one possible route is to partially hydrogenate the alkyne to an alkene and then perform a reaction that introduces more double bonds into the carbon chain
3
Step 1 - Partial Hydrogenation: Use a catalyst such as Lindlar's catalyst to partially hydrogenate the alkyne to a cis-alkene. The reaction would be:
4
Step 2 - Formation of additional double bonds: Perform an isomerization reaction to spread the double bonds along the carbon chain. This could be achieved using a strong base such as sodium amide (). The reaction would be:
Answer
Step 1: Partially hydrogenate the alkyne using Lindlar's catalyst to obtain a cis-alkene. Step 2: Treat the cis-alkene with sodium amide to isomerize and form the poly-alkene with multiple double bonds.
Key Concept
Synthesis of poly-alkenes from alkynes
Explanation
The process involves partial hydrogenation of an alkyne to form a cis-alkene, followed by isomerization to spread the double bonds along the carbon chain.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question
