
Sia
6. The following reaction scheme was allowed to reach chemical equilibrium:
Here
the 7th digit of your URN
a) Initially the system contained no products and of . Find the equilibrium composition of the reactor.
b) Given an exothermic forward reaction explain how (and why) you could improve the production of and (max 200 words)
c) Given that forward reaction is exothermic, explain with justification how an increase in temperature affects the values of and ? (max 200 words)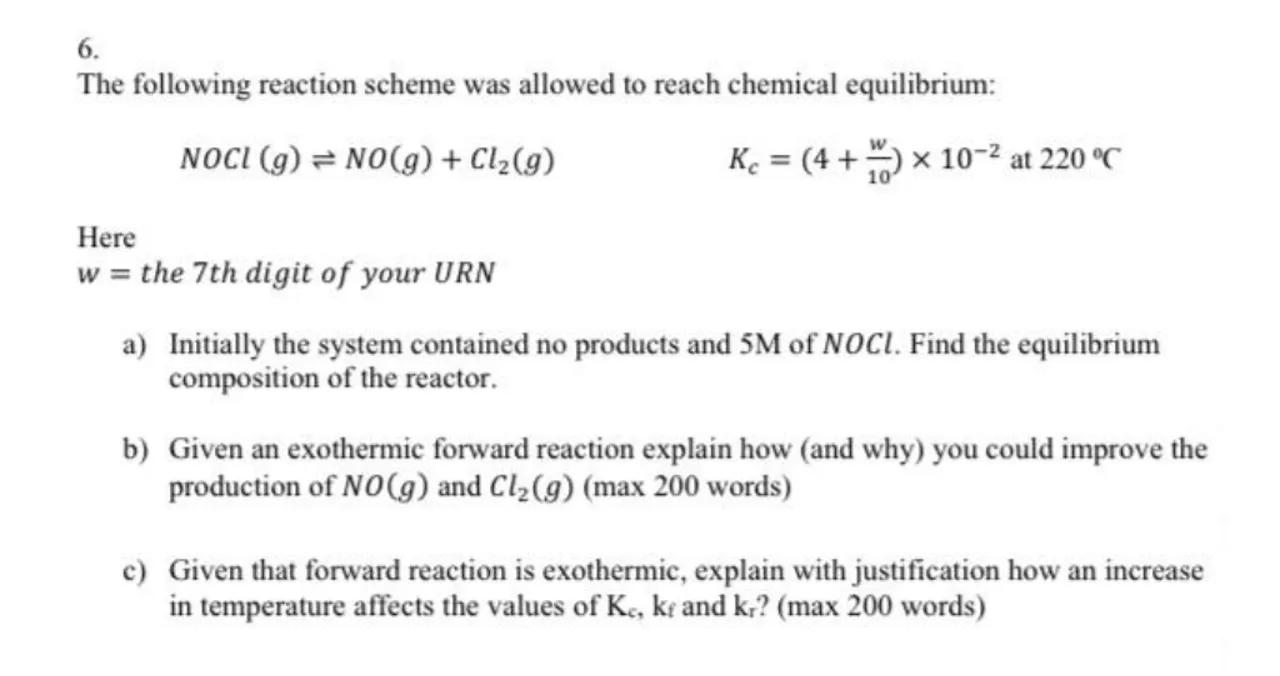

Answer from Sia
Posted almost 2 years ago
Solution
1
Set up the ICE table: To find the equilibrium composition, we use an ICE (Initial, Change, Equilibrium) table. Initially, we have 5M of NOCl and 0M of NO and Cl₂. Let the change in concentration of NOCl be and the formation of NO and Cl₂ be each
2
Write the equilibrium expression: The equilibrium constant expression for the reaction is . Substituting the equilibrium concentrations from the ICE table gives us
3
Calculate the equilibrium concentrations: Given , we solve for using the quadratic formula. This will give us the equilibrium concentrations of NOCl, NO, and Cl₂
1 Answer
[Insert calculated equilibrium concentrations here]
Key Concept
Chemical equilibrium and the use of ICE tables to find equilibrium concentrations
Explanation
The ICE table method allows us to express the changes in concentration of reactants and products in terms of a single variable, , which we can solve for using the equilibrium constant.
Solution
1
Understand Le Chatelier's Principle: To improve the production of NO and Cl₂, we can apply Le Chatelier's Principle, which states that a system at equilibrium will adjust to counteract any imposed change
2
Apply changes to the system: Since the forward reaction is exothermic, decreasing the temperature will shift the equilibrium to the right, producing more NO and Cl₂. Removing the products as they are formed will also shift the equilibrium to the right
2 Answer
Decrease temperature and remove products to improve production of NO and Cl₂
Key Concept
Le Chatelier's Principle and its application to chemical equilibria
Explanation
By decreasing temperature and removing products, we can shift the equilibrium towards the products in an exothermic reaction, increasing their production.
Solution
1
Relate temperature to equilibrium constants: An increase in temperature for an exothermic reaction will decrease the value of due to Le Chatelier's Principle
2
Understand the effect on rate constants: The Arrhenius equation shows that as temperature increases, the rate constants for both the forward and reverse reactions ( and ) will increase, but will increase more for an exothermic reaction
3 Answer
Increase in temperature decreases , increases both and , but increases more
Key Concept
Temperature's effect on equilibrium constants and rate constants
Explanation
For exothermic reactions, increasing temperature decreases the equilibrium constant and increases the rate constants, with a greater increase in the reverse rate constant.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question