
Sia
6) This question is concerned with vibrational spectra (Infrared and Raman).
a) How many normal modes of vibration are expected in these molecules? (Show calculations)
i) Theobromine (the caffeine-like stimulant that is found in chocolate)
ii) Cyanopolyyne (an organic molecular wire that has been detected spectroscopically in interstellar space, and is postulated to be a precursor to biomolecules:
b) In the molecular vibration shown here for trans-1,4-dichlorocyclohexane, both chlorine atoms stretch away from the carbon to which they are bonded (arrows). Will this mode be IR active? Will this mode be Raman active? In each case, why or why not?
c) Why are and considered greenhouse gases, but and are not?
d) Are the vibrations shown here a degenerate pair (Yes/No)? Why or why not?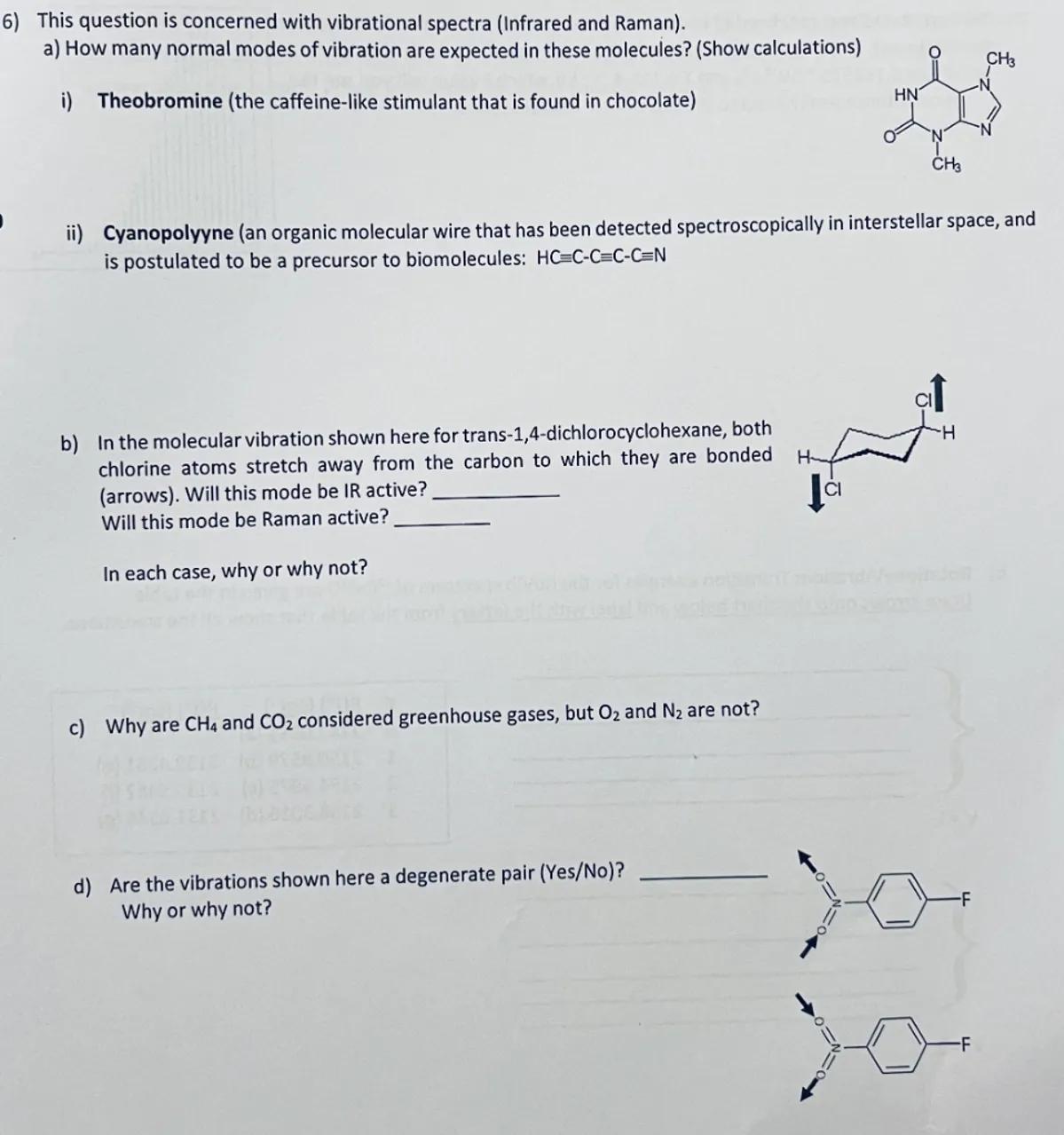

Answer from Sia
Posted almost 2 years ago
Solution
1
Calculate the number of normal modes for Theobromine: The number of normal modes of vibration for a molecule can be calculated using the formula for non-linear molecules, where is the number of atoms in the molecule
2
Calculate the number of normal modes for Cyanopolyyne: For linear molecules, the formula is . Cyanopolyyne is a linear molecule, so we will use this formula to find the number of normal modes
3
Determine IR and Raman activity for trans-1,4-dichlorocyclohexane: A vibrational mode is IR active if it results in a change in the dipole moment of the molecule. It is Raman active if there is a change in polarizability
4
Explain why $\mathrm{CH}_{4}$ and $\mathrm{CO}_{2}$ are greenhouse gases: Greenhouse gases absorb and emit infrared radiation due to their molecular vibrations, which involve changes in dipole moment. Molecules like and do not have this property because they are homonuclear diatomic molecules with no permanent dipole moment
5
Determine if the vibrations are a degenerate pair: Degenerate vibrations are those that have the same energy but occur in different spatial directions. We need to examine the symmetry and energy of the vibrations to answer this
1 Answer
The number of normal modes for Theobromine and Cyanopolyyne needs to be calculated using the respective formulas for non-linear and linear molecules.
2 Answer
The vibrational mode for trans-1,4-dichlorocyclohexane is IR active if there is a change in dipole moment and Raman active if there is a change in polarizability.
3 Answer
and are greenhouse gases because they can absorb and emit infrared radiation, unlike and , which cannot due to their lack of a permanent dipole moment.
4 Answer
Whether the vibrations are a degenerate pair depends on their symmetry and energy.
Key Concept
Normal modes of vibration
Explanation
The number of normal modes of vibration depends on whether the molecule is linear or non-linear and is calculated using the formulas or , respectively.
Key Concept
IR and Raman activity
Explanation
A vibrational mode is IR active if it changes the dipole moment and Raman active if it changes the polarizability of the molecule.
Key Concept
Greenhouse effect
Explanation
Greenhouse gases absorb and emit infrared radiation due to changes in their dipole moment during molecular vibrations, which is not possible for homonuclear diatomic molecules like and .
Key Concept
Degenerate vibrations
Explanation
Degenerate vibrations are those with the same energy occurring in different spatial directions, often associated with the molecule's symmetry.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question