
Sia
7) The compound decomposes to and at elevated temperatures. Find the rate law for this reaction from the following rate data.
\begin{tabular}{ll}
P & Initial rate (atm/sec) \\
& \\
& \\
&
\end{tabular}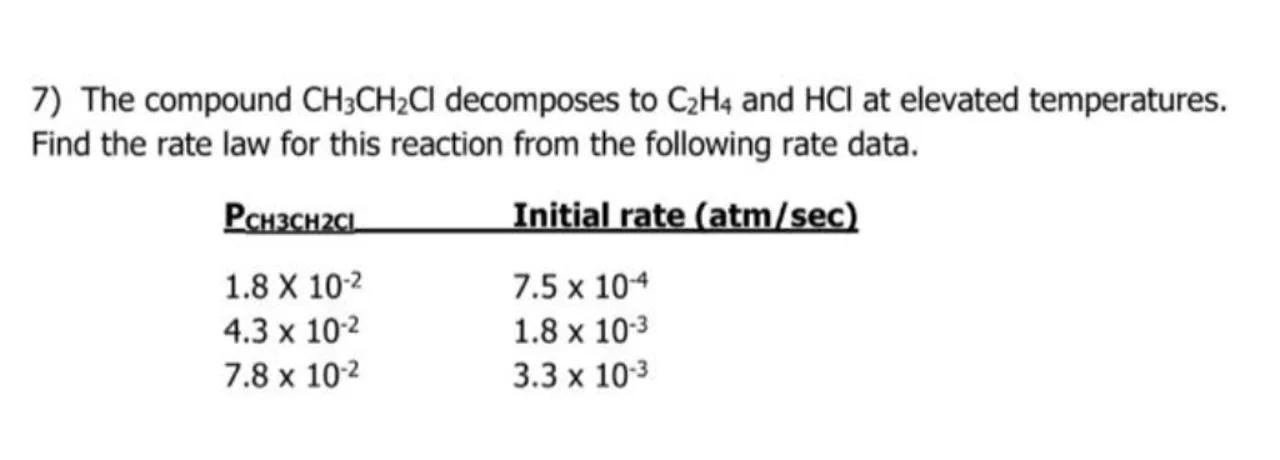

Answer from Sia
Posted almost 2 years ago
Solution
1
Identify the reaction: The given reaction is the decomposition of to and
2
Write the general rate law: The rate law for the reaction can be written as , where is the rate constant and is the order of the reaction with respect to
3
Use the given data to determine the order of the reaction:
- For atm, the initial rate is atm/sec.
- For atm, the initial rate is atm/sec.
- For atm, the initial rate is atm/sec.
To find , we can use the ratio of the rates and the concentrations:
Using the first two data points:
Taking the logarithm of both sides:
Thus, the reaction is first order with respect to
4
Write the rate law: The rate law for the reaction is
Answer
The rate law for the decomposition of is .
Key Concept
Rate law determination
Explanation
The rate law expresses the relationship between the rate of a chemical reaction and the concentration of its reactants. By comparing the initial rates and concentrations, we determined that the reaction is first order with respect to .
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question