
Sia

Question
Chemistry
Posted over 1 year ago
A lab group is given 25.0 grams of an unknown metal. The students are instructed to identify the metal by determining its specific heat capacity. Their unknown must be one of the metals from the following table:
\begin{tabular}{lc}
Substance & Specific heat \\
\hline Aluminum & 0.897 \\
Magnesium & 1.024 \\
Silver & 0.235 \\
Zinc & 0.388
\end{tabular}
Using a standard calorimetry set-up and three separate trials, the students report that their sample released -1760 joules of thermal energy when it cooled from to .
Specific heat equation
What is the likely identity of the metal?
Choose 1 answer:
(A) aluminum
(B) magnesium
(c) silver
(D) zinc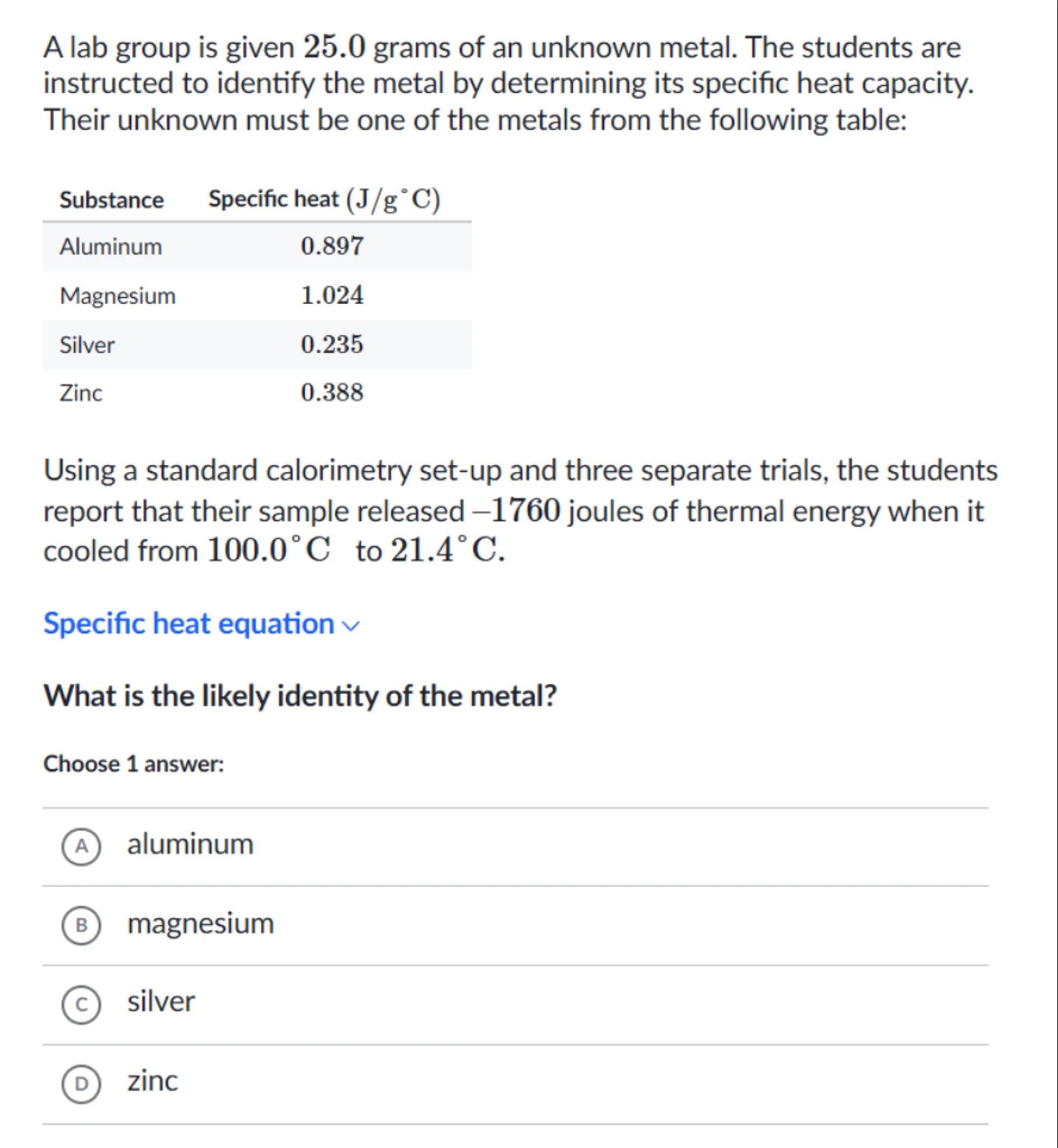

Answer from Sia
Posted over 1 year ago
D
Key Concept
Specific Heat Capacity
Explanation
The specific heat capacity can be calculated using the formula . Given the thermal energy released ( J), mass ( g), and temperature change (), we can solve for the specific heat capacity (). The calculated specific heat capacity is closest to that of zinc (0.388 J/g°C).
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question