
Sia
A steel cylinder contains 5.00 mole of graphite (pure carbon) and 5.00 moles of . The mixture is ignited and all the graphite reacts. Combustion produces a mixture of gas and gas. After the cylinder has cooled to its original temperature, it is found that the pressure of the cylinder has increased by . Calculate the mole fractions of , and in the final gaseous mixture.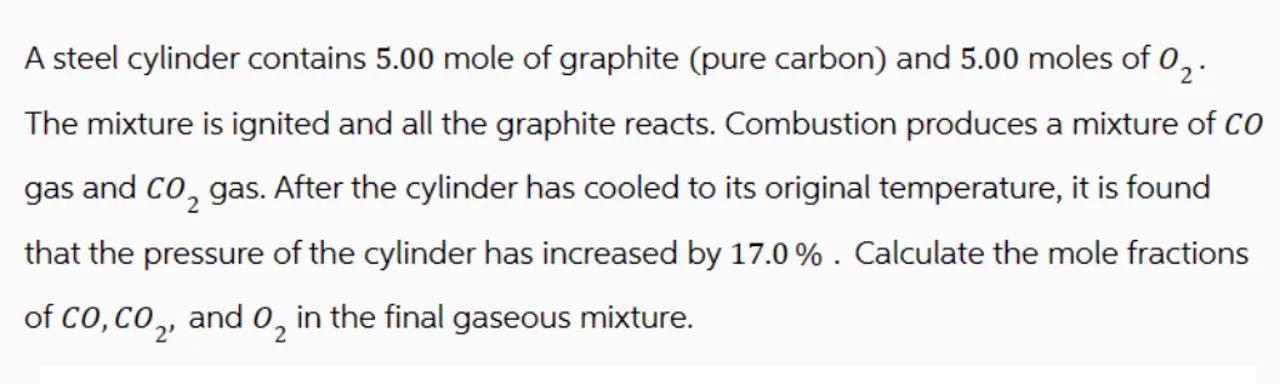

Answer from Sia
Posted almost 2 years ago
Solution
1
Write the balanced chemical equations: The combustion of carbon (graphite) can produce either carbon monoxide () or carbon dioxide (). The two possible reactions are: and
2
Determine the limiting reactant: Since we have equal moles of and , and the reaction to form requires a 1:1 mole ratio, while the reaction to form requires a 2:1 mole ratio of to , is the limiting reactant for both reactions
3
Calculate the moles of gases after reaction: If all is used to form , we would have 5 moles of . If all is used to form , we would have 10 moles of . Since the pressure increased by 17%, we know that the total moles of gas increased. This means that some must have been used to form . Let be the moles of that reacted to form , then is the moles of that reacted to form . The total moles of gas after reaction is
4
Relate the change in moles to change in pressure: Assuming ideal gas behavior and constant temperature and volume, the pressure is proportional to the number of moles of gas. Let be the original pressure, then the final pressure is . The initial moles of gas is 10 (5 moles of and 5 moles of ), so . Solving for gives moles
5
Calculate the mole fractions: The mole fraction of a component is the number of moles of that component divided by the total number of moles. The mole fraction of is , the mole fraction of is , and the mole fraction of is . Plugging in gives the mole fractions
Answer
Mole fraction of :
Mole fraction of :
Mole fraction of :
Key Concept
The mole fraction of a component in a mixture is the ratio of the number of moles of that component to the total number of moles of all components in the mixture.
Explanation
The mole fractions were calculated based on the stoichiometry of the combustion reactions and the given increase in pressure, which indicates the total moles of gas after the reaction. By assuming ideal gas behavior, the change in pressure directly relates to the change in the number of moles of gas.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question
