
Sia
Application
Name:
Date:
\begin{tabular}{|l|l|l|l|l|l|}
\hline & & & & \\
\hline Lewis Diagram & & & & \\
\\
\hline
\end{tabular}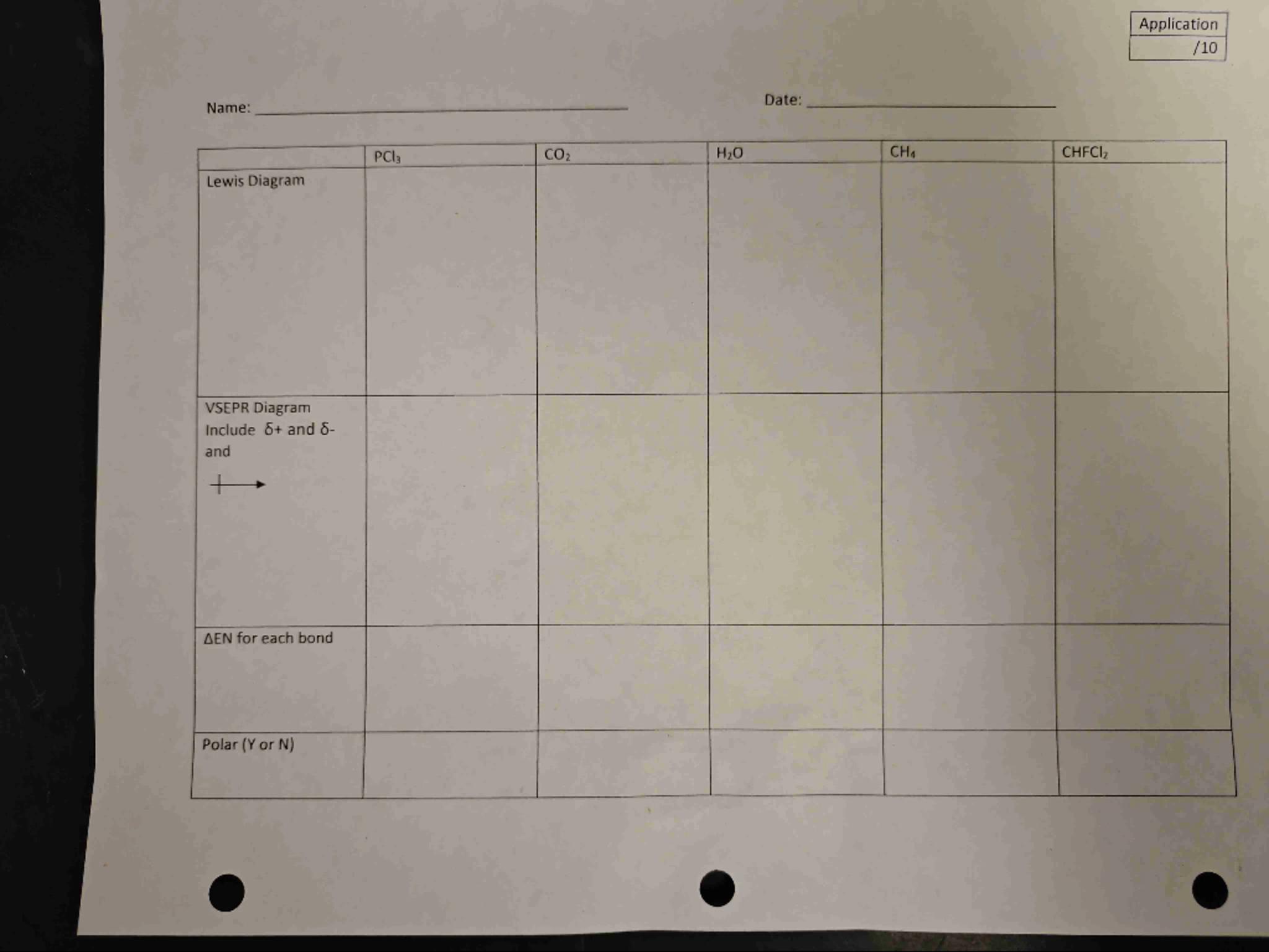

Answer from Sia
Posted over 1 year ago
Hi Violet81697! It looks like you have a table related to some chemistry applications, specifically focusing on molecular structures and properties.
To fill out the table, you need to provide the Lewis diagrams, VSEPR diagrams, electronegativity differences (ΔEN) for each bond, and determine if each molecule is polar or not.
Here's a brief overview of what you need to do for each molecule:
1. Lewis Diagram: Draw the Lewis structure for each molecule. This involves showing the arrangement of atoms and the bonding electrons.
2. VSEPR Diagram: Use the VSEPR theory to determine the molecular geometry of each molecule. This will help you visualize the shape based on the number of bonding and lone pairs of electrons.
3. ΔEN for each bond: Calculate the electronegativity difference for each bond in the molecule. You can find the electronegativity values on the periodic table. The formula is:
4. Polar (Y or N): Determine if the molecule is polar or nonpolar based on the symmetry of the molecule and the electronegativity differences. If the molecule has polar bonds and is asymmetrical, it is likely polar (Y). If it is symmetrical, it is likely nonpolar (N).
If you need help with any specific molecule or part of the table, feel free to ask!Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question