
Sia

Question
Chemistry
Posted over 1 year ago
Complete the statement by entering a number.
A neutral Se atom has valence electron(s).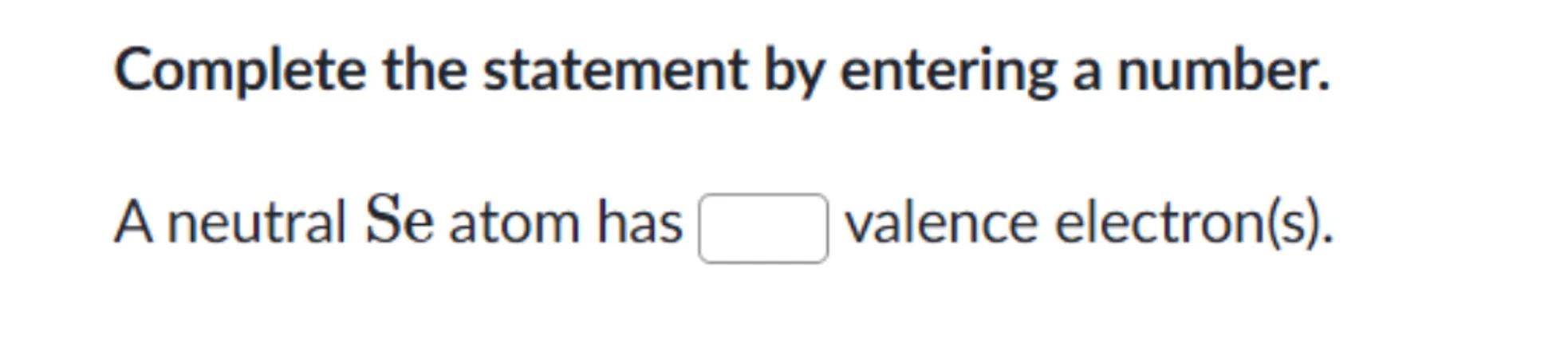

Answer from Sia
Posted over 1 year ago
Solution
1
Identify the element: Selenium (Se) is the element in question
2
Determine the group: Selenium is in Group 16 of the periodic table
3
Count the valence electrons: Elements in Group 16 have 6 valence electrons
Answer
6
Key Concept
Valence electrons in Group 16 elements
Explanation
Elements in Group 16 of the periodic table, including Selenium (Se), have 6 valence electrons.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question