
Sia
Draw the organic product of the following reaction.
- You do not have to consider stereochemistry.
- You do not have to explicitly draw atoms.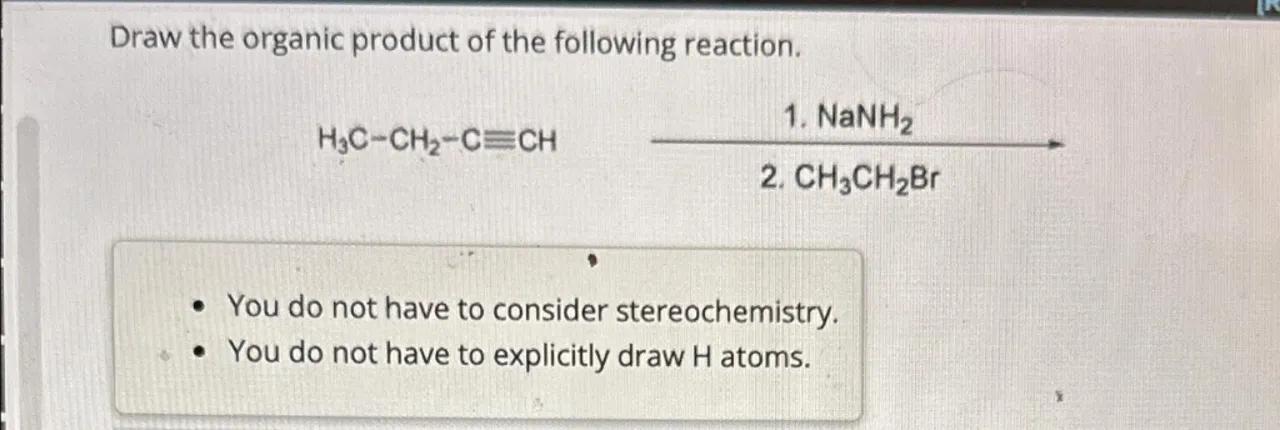

Answer from Sia
Posted almost 2 years ago
Solution
1
Identify the starting material: The organic compound described is 1-butyne, which has the structure
2
First reaction step with NaNH2: Sodium amide, NaNH2, is a strong base that will deprotonate the terminal alkyne, creating a sodium acetylide anion. The reaction is
3
Second reaction step with CH3CH2Br: The sodium acetylide anion will act as a nucleophile and attack the primary alkyl halide, bromoethane, in an SN2 reaction, displacing the bromide ion and forming a new carbon-carbon bond. The reaction is
Answer
The organic product of the following reaction is pent-1-yne, .
Key Concept
Alkyne deprotonation and subsequent nucleophilic substitution
Explanation
A terminal alkyne is deprotonated by a strong base to form an acetylide anion, which then acts as a nucleophile in an SN2 reaction with a primary alkyl halide to form a new carbon-carbon bond.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question
