
Sia
Draw the organic product of the following reaction.
- You do not have to consider stereochemistry.
- You do not have to explicitly draw atoms.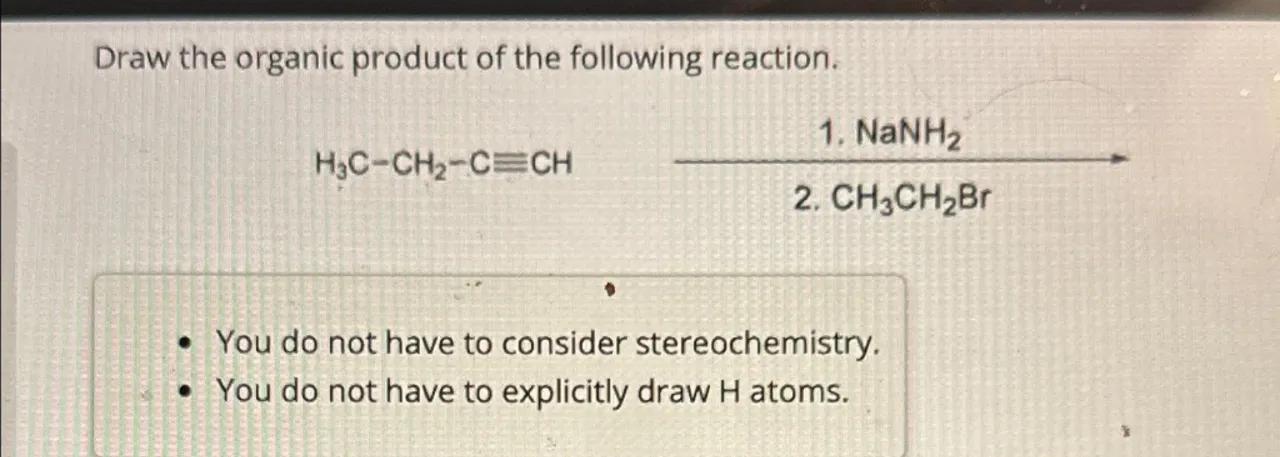

Answer from Sia
Posted almost 2 years ago
Solution
1
Identify the starting material: The starting material is a 4-carbon alkyne with the triple bond between the second and third carbons, known as 1-butyne
2
First reaction step with NaNH2: NaNH2 is a strong base that will deprotonate the terminal alkyne, creating a negatively charged acetylide ion on the first carbon. The reaction is as follows:
3
Second reaction step with CH3CH2Br: The acetylide ion is a good nucleophile and will attack the primary alkyl halide, CH3CH2Br, in an S_N2 reaction, displacing the bromide ion and forming a new carbon-carbon bond. The reaction is as follows:
Answer
The organic product of the following reaction is pent-1-yne (HC≡C-CH2-CH2-CH3).
Key Concept
Acetylide ion formation and nucleophilic substitution
Explanation
The acetylide ion formed from deprotonation of a terminal alkyne reacts with a primary alkyl halide in an S_N2 reaction to form a new carbon-carbon bond, extending the carbon chain.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question
