
Sia
For the questions below please indicate the expected major product for the indicated reaction. Please indicate the major resonance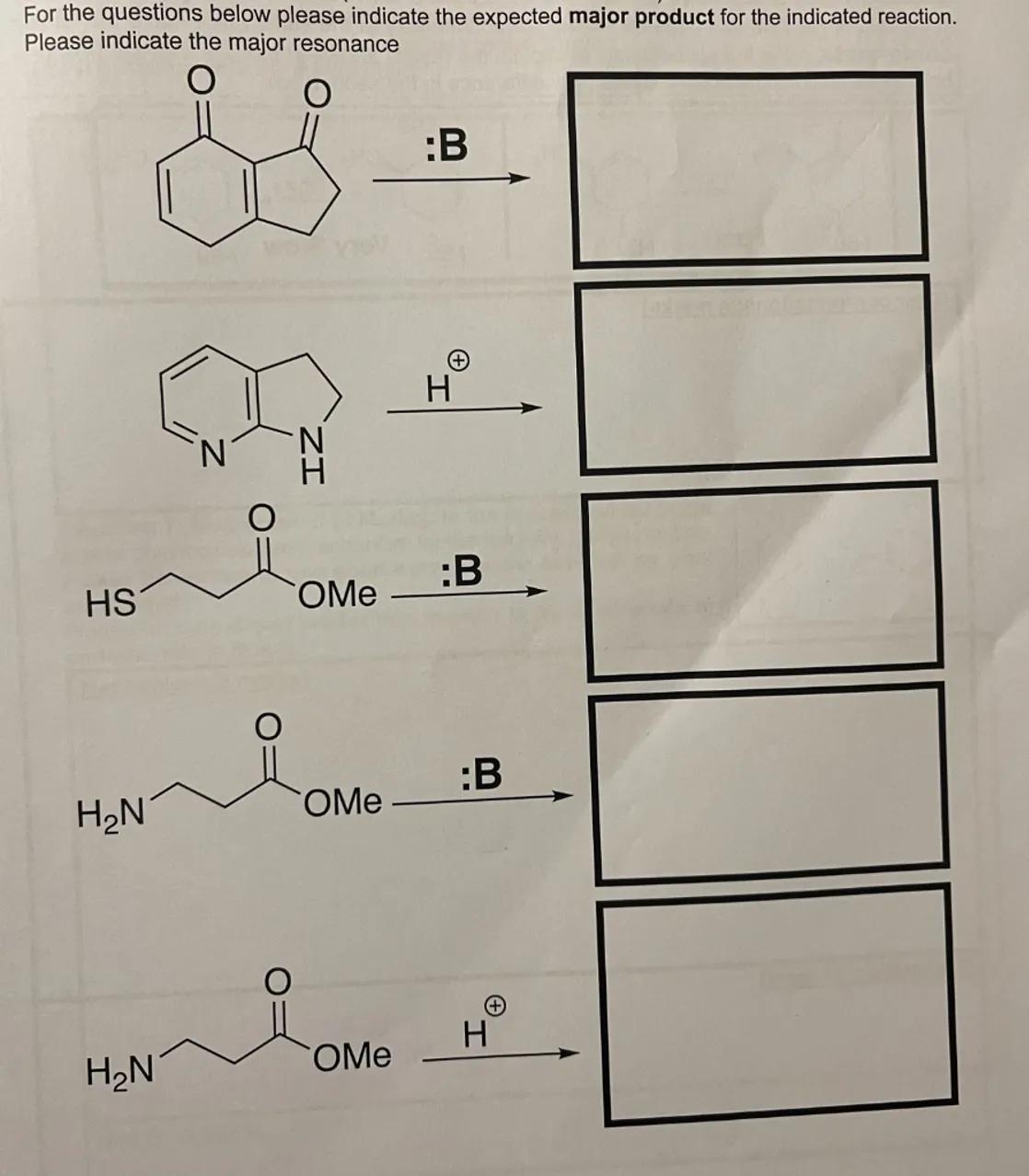

Answer from Sia
Posted almost 2 years ago
Solution
1
Identify the reaction for the bicyclic ketone compound: The bicyclic compound with two ketone groups will react with borane (":B") in a reduction process
2
Predict the major product for reaction 1: The borane will reduce the ketone groups to secondary alcohols. The bicyclic compound will have two hydroxyl groups (-OH) where the ketone groups were originally located
3
Identify the reaction for the heterocyclic compound: The heterocyclic compound with two nitrogen atoms will react with borane. This reaction is less common, but borane can potentially reduce imine or nitrile groups if present
4
Predict the major product for reaction 2: Without specific details on the functional groups attached to the nitrogen atoms, a general prediction is challenging. However, if reducible groups are present, borane would reduce them to amines
5
Identify the reaction for the thiol-ester compound: The molecule containing a thiol (SH) group and an ester (OMe) group will react with borane. Borane typically reduces carbonyl compounds, not thiols or esters
6
Predict the major product for reaction 3: The borane will not react with the thiol or ester group under normal conditions, so no reaction is expected
7
Identify the reaction for the amine-ester compound with borane: The molecule containing an amine (NH2) group and an ester (OMe) group will react with borane. Borane might reduce the ester to an alcohol if the conditions are suitable
8
Predict the major product for reaction 4: The borane could reduce the ester group to an alcohol, leaving the amine group untouched
9
Identify the reaction for the amine-ester compound with a proton: The same molecule as in reaction 4, but reacting with a proton (H⊕), will undergo an acid-base reaction
10
Predict the major product for reaction 5: The amine group will be protonated, forming an ammonium ion. The ester group will remain unchanged
1 Answer
The bicyclic ketone compound will be reduced to a bicyclic diol.
2 Answer
The heterocyclic compound's reaction with borane is uncertain without more information on the functional groups.
3 Answer
No reaction is expected for the thiol-ester compound with borane.
4 Answer
The amine-ester compound will be reduced by borane to form an alcohol from the ester group, while the amine remains unchanged.
5 Answer
The amine group in the amine-ester compound will be protonated to form an ammonium ion when reacting with a proton.
Key Concept
Borane is a reducing agent that typically reduces carbonyl compounds to alcohols.
Explanation
Borane selectively reduces carbonyl groups in organic compounds, converting ketones to secondary alcohols. It does not typically react with esters or thiols under normal conditions.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question
