
Sia
Give IUPAC names for the following compounds:
(a)
(b)
Submit Answer
Try Another Version
attempts remaining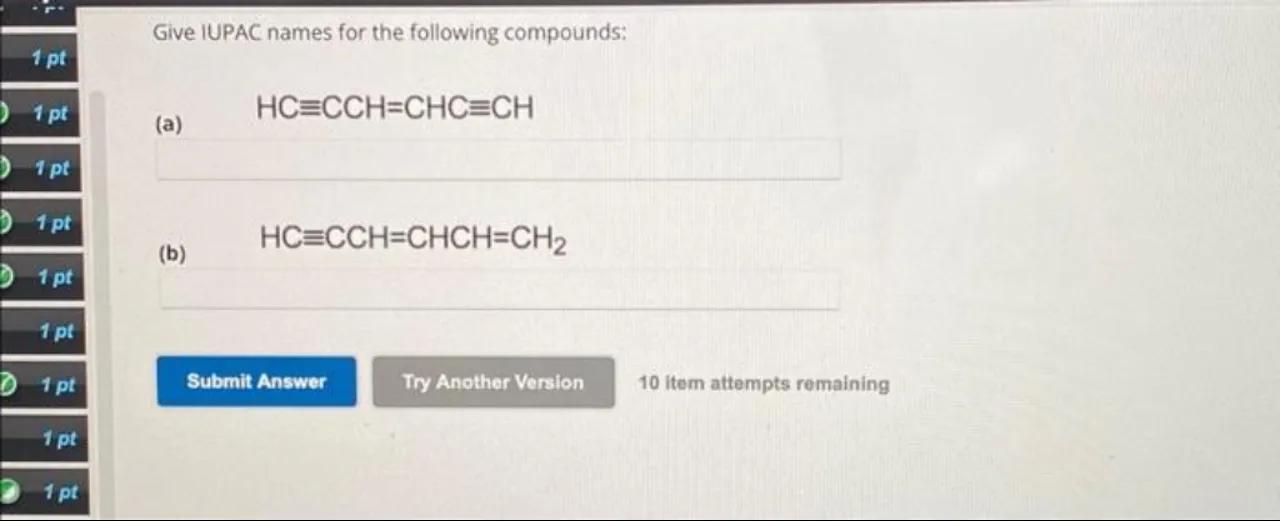

Answer from Sia
Posted almost 2 years ago
Solution
1
Identify the structure: The first compound (a) is given as . This structure contains two triple bonds and one double bond
2
Number the carbon chain: Number the longest carbon chain that includes the triple and double bonds. Start from the end nearest to the first multiple bond. For compound (a), the numbering starts from the left:
3
Identify the position of the bonds: The triple bonds are at positions 1 and 4, and the double bond is at position 3
4
Name the compound: The IUPAC name is derived by combining the positions of the multiple bonds with the base name of the longest chain. For compound (a), the name is 1,4-Hexadiyne-3-ene
1
Identify the structure: The second compound (b) is given as . This structure contains one triple bond and two double bonds
2
Number the carbon chain: Number the longest carbon chain that includes the triple and double bonds. Start from the end nearest to the first multiple bond. For compound (b), the numbering starts from the left:
3
Identify the position of the bonds: The triple bond is at position 1, and the double bonds are at positions 3 and 5
4
Name the compound: The IUPAC name is derived by combining the positions of the multiple bonds with the base name of the longest chain. For compound (b), the name is 1-Hexyne-3,5-diene
Answer
(a) 1,4-Hexadiyne-3-ene
(b) 1-Hexyne-3,5-diene
Key Concept
The IUPAC naming of organic compounds involves identifying the longest carbon chain containing the highest order multiple bonds and numbering it to give the lowest possible numbers to the multiple bonds.
Explanation
For compound (a), the longest chain has six carbons with triple bonds at positions 1 and 4, and a double bond at position 3, leading to the name 1,4-Hexadiyne-3-ene. For compound (b), the longest chain has six carbons with a triple bond at position 1 and double bonds at positions 3 and 5, leading to the name 1-Hexyne-3,5-diene.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question