
Sia
Identify the major product(s) in the reaction of 2 -bromo-3ethyipentane with sodium hydroxide.
I
II
III
N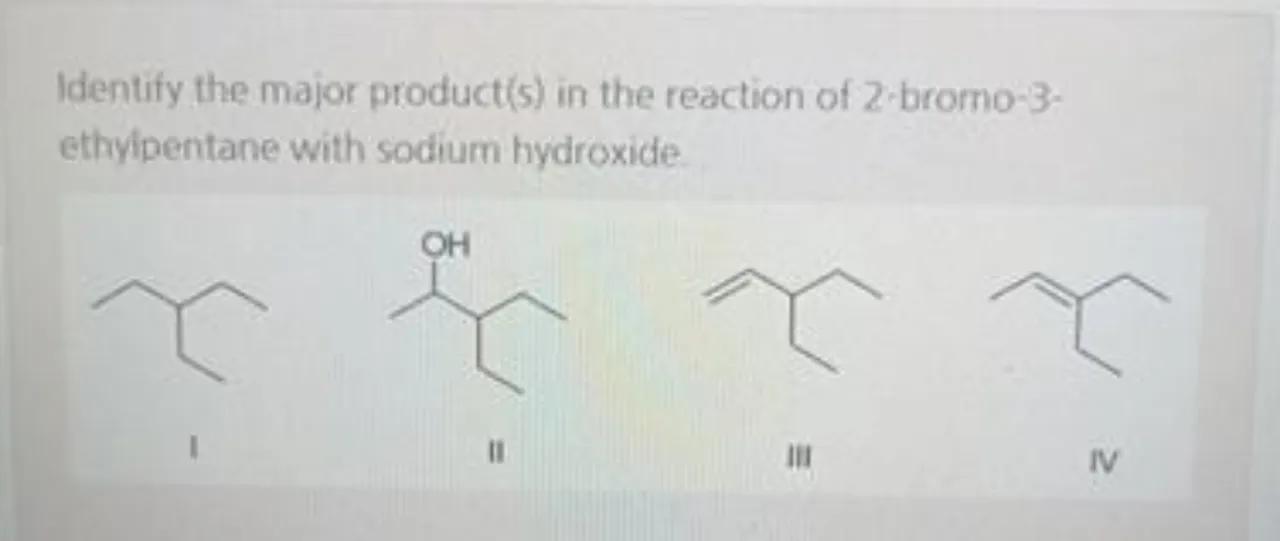

Answer from Sia
Posted almost 2 years ago
B
Key Concept
Nucleophilic Substitution Reactions (SN2)
Explanation
The reaction of 2-bromo-3-ethylpentane with sodium hydroxide (NaOH) is a nucleophilic substitution reaction (SN2). In this reaction, the hydroxide ion (OH⁻) from NaOH acts as a nucleophile and attacks the carbon atom that is bonded to the bromine atom, resulting in the displacement of the bromine and formation of a new carbon-oxygen bond. The major product will be the one where the hydroxide ion has attacked the carbon atom at the position where the bromine was originally attached, leading to the formation of an alcohol. Since the reaction proceeds via an SN2 mechanism, the hydroxide ion attacks from the opposite side of the leaving group, resulting in an inversion of configuration at the carbon atom. Product II corresponds to this description, where the OH group is attached to the second carbon atom, which is the position previously occupied by the bromine atom
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question