
Sia

Question
Math
Posted almost 2 years ago
If a beaker has , then the beaker has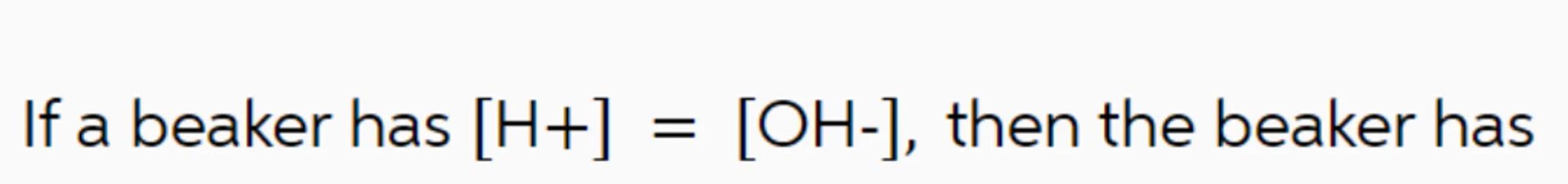

Answer from Sia
Posted almost 2 years ago
Solution by Steps
step 1
Determine the nature of the solution in the beaker based on the concentration of hydrogen ions and hydroxide ions
step 2
If , then the solution is neutral. This is a characteristic of pure water at 25°C (298 K)
step 3
The neutrality of the solution is due to the equal concentration of hydrogen and hydroxide ions, which is a fundamental concept in acid-base chemistry
Answer
The beaker has a neutral solution.
Key Concept
Neutral Solution
Explanation
A solution is neutral when the concentrations of hydrogen ions and hydroxide ions are equal, which typically occurs in pure water at standard conditions.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question