
Sia
In the figure below, what is the pressure of the gas in the flask (in atm) if the barometer reads 693.4 torr? Round your answer to 4 significant figures.
Note: Reference the SI prefixes and Conversion factors for non-SI units tables for additional information.
Pressure atm 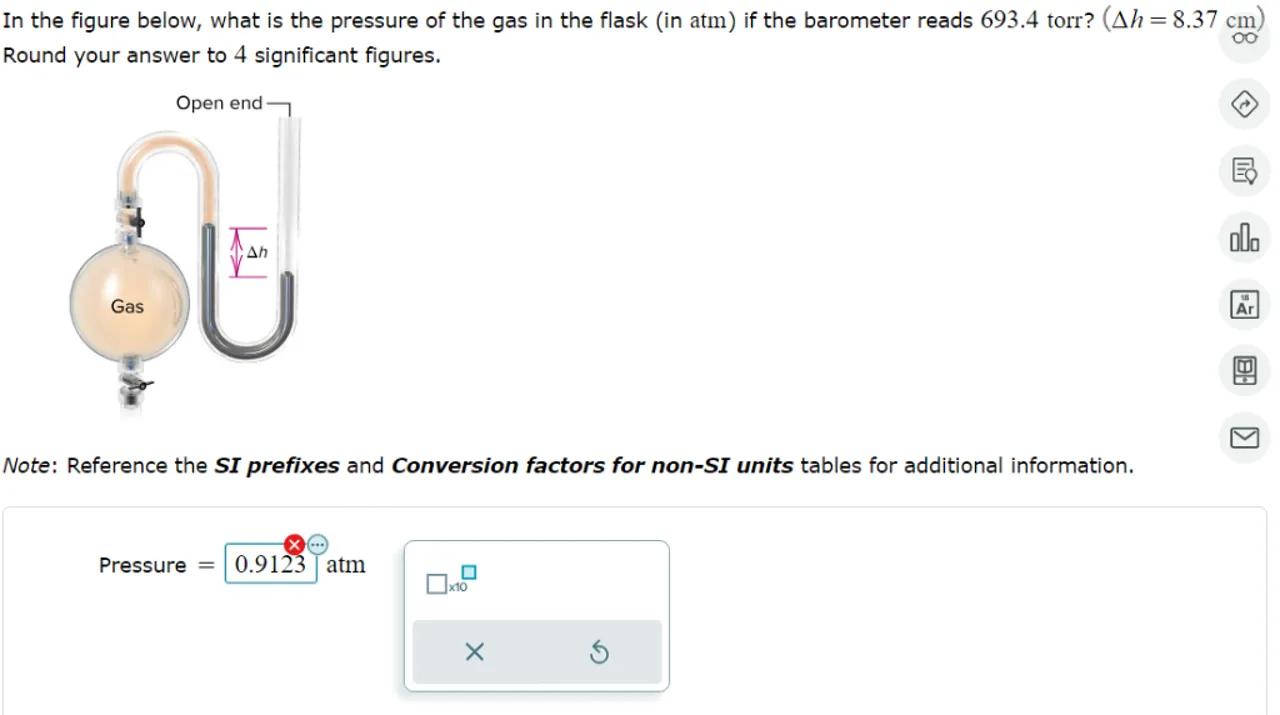

Answer from Sia
Posted almost 2 years ago
A
Key Concept
Pressure Conversion and Manometer Reading
Explanation
To find the pressure of the gas in the flask, we need to convert the barometric pressure from torr to atm and account for the pressure difference indicated by the manometer. The conversion factor from torr to atm is . The pressure difference due to the height of the liquid column in the manometer is in cm of liquid, which must be converted to atm. The total pressure of the gas is the atmospheric pressure minus the pressure difference if the liquid level on the side open to the atmosphere is higher.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question