
Sia
Indicate whether the pair of structures shown represent stereoisomers, constitutional isomers, different conformations o same compound, or the same conformation of a compound viewed from a different perspective.
Note that cis, trans isomers are an example of stereoisomers.
Submit Answer
Retry Entire Group
6 more group attempts remaining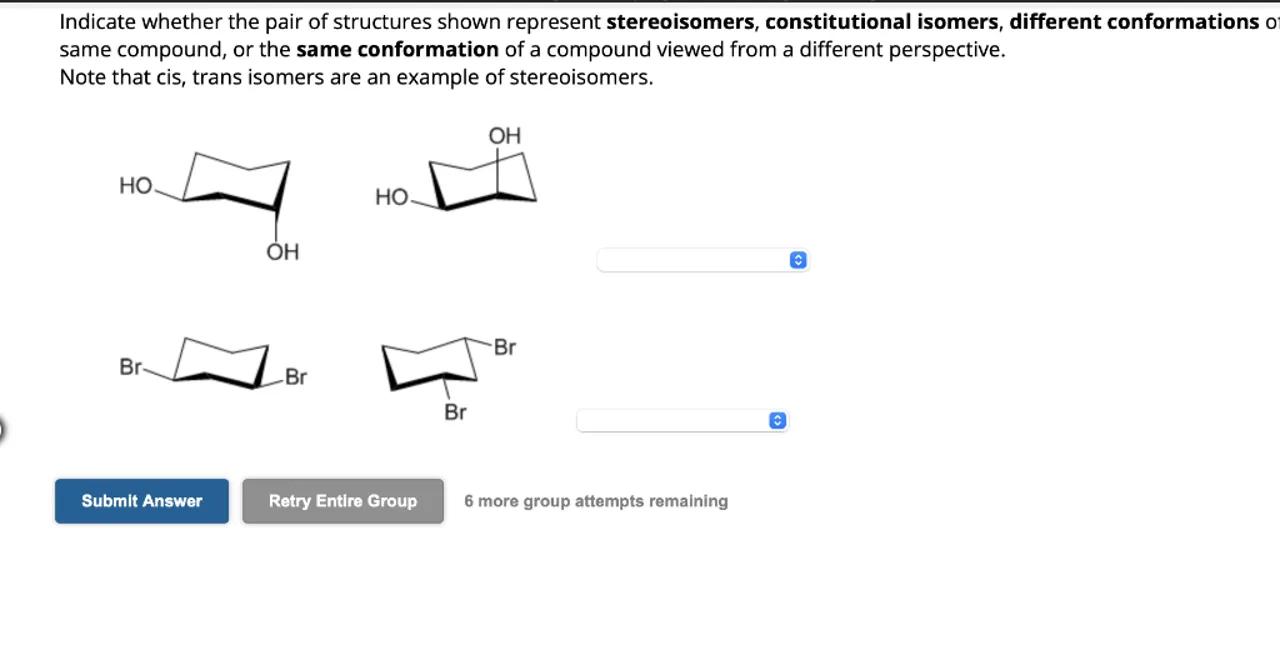

Answer from Sia
Posted almost 2 years ago
Solution
1
Identify the type of isomerism: The first set of molecules has a six-membered ring with two OH groups and one CH2OH group attached. The difference in orientation of these groups suggests that these molecules are stereoisomers
2
Analyze the second set of molecules: The second set of molecules has a six-membered ring with two Br atoms attached. The difference in the orientation of the Br atoms indicates that these molecules are also stereoisomers
Answer
Both sets of molecules represent stereoisomers.
Key Concept
Stereoisomers
Explanation
Stereoisomers are compounds that have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question