
Sia

Question
Chemistry
Posted over 1 year ago
Please design the synthetic route of the following transformation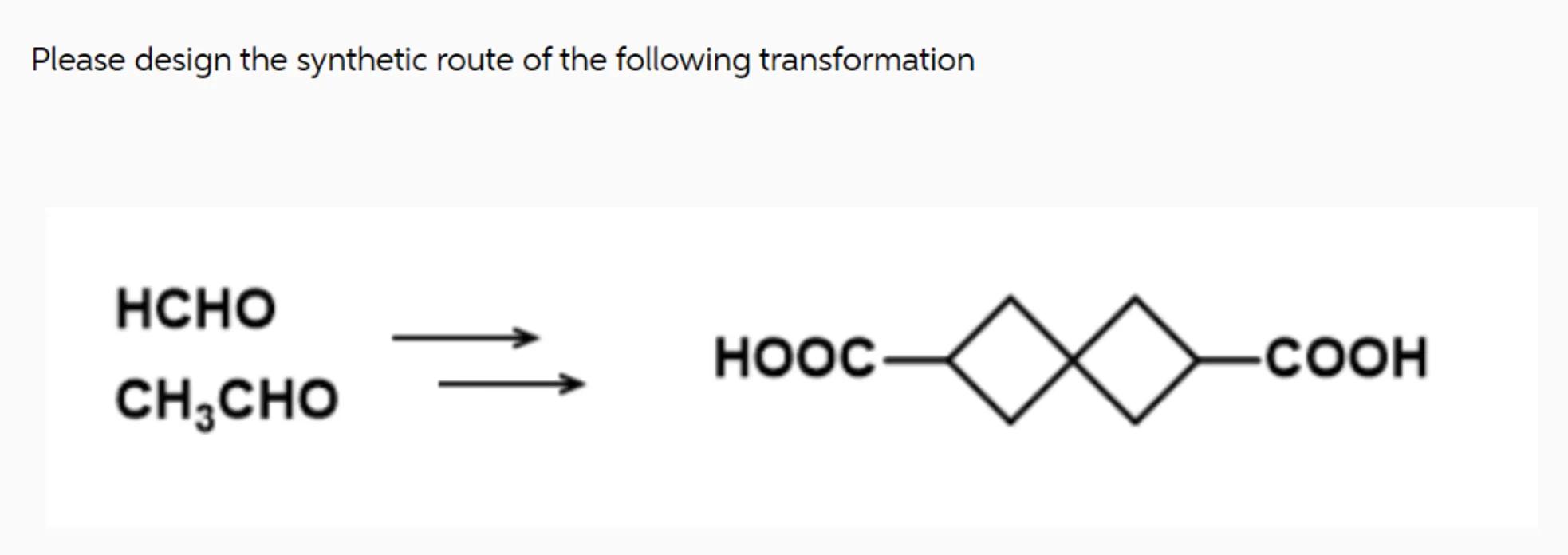

Answer from Sia
Posted over 1 year ago
Solution
1
Identify the starting materials: The starting materials are formaldehyde (HCHO) and acetaldehyde (CH3CHO)
2
Determine the target molecule: The target molecule consists of two four-membered rings connected by a single bond, with a HOOC group attached to the leftmost carbon of the left ring and a COOH group attached to the rightmost carbon of the right ring
3
Plan the synthetic route:
- Step 1: Perform an aldol condensation between HCHO and CH3CHO to form a β-hydroxy aldehyde.
- Step 2: Dehydrate the β-hydroxy aldehyde to form an α,β-unsaturated aldehyde.
- Step 3: Cyclize the α,β-unsaturated aldehyde to form the first four-membered ring.
- Step 4: Perform a similar sequence of reactions to form the second four-membered ring.
- Step 5: Connect the two four-membered rings via a single bond.
- Step 6: Introduce the carboxylic acid groups (HOOC and COOH) at the appropriate positions
Answer
The synthetic route involves aldol condensation, dehydration, cyclization, and functional group introduction steps.
Key Concept
Aldol condensation and cyclization are key steps in forming complex ring structures.
Explanation
Aldol condensation between formaldehyde and acetaldehyde forms a β-hydroxy aldehyde, which upon dehydration forms an α,β-unsaturated aldehyde. This intermediate can then undergo cyclization to form the desired ring structures, followed by functional group modifications to introduce the carboxylic acid groups.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question