
Sia

Question
Chemistry
Posted over 1 year ago
Predict the products of the following reactions. If the reaction is an acid-base reaction, predict its position of equilibrium.
(a) (b)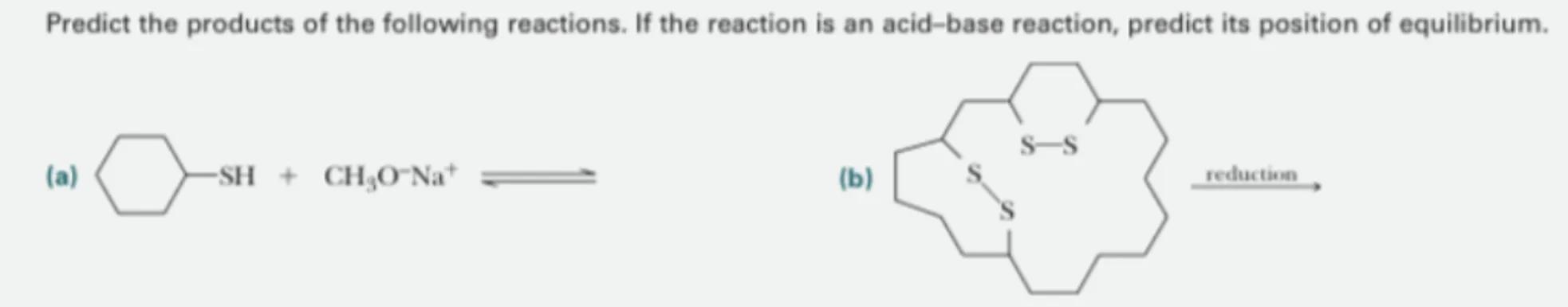

Answer from Sia
Posted over 1 year ago
Sure, let's analyze the given reactions and predict the products.
Reaction (a)
The first reaction involves a six-membered cyclic structure with an SH group reacting with . This is an acid-base reaction where the SH group (thiol) acts as an acid and (methoxide) acts as a base.
The reaction can be represented as:
The thiol group donates a proton to the methoxide ion, forming a thiolate anion and methanol.
Reaction (b)
The second reaction involves a 12-membered cyclic structure with three sulfur atoms undergoing reduction. Reduction typically involves the gain of electrons or hydrogen atoms.
Without specific details on the reducing agent, a common reduction reaction for sulfur-containing compounds is the reduction of disulfide bonds () to thiols ().
The reaction can be represented as:
Position of Equilibrium
For the acid-base reaction in (a), the position of equilibrium can be predicted based on the relative strengths of the acids and bases involved. Methanol () is a weaker acid compared to the thiol group, so the equilibrium will favor the formation of methanol and the thiolate anion.
Answer Format
For the given questions:
(a) D
Key Concept
Acid-Base Reaction
Explanation
The SH group donates a proton to the methoxide ion, forming a thiolate anion and methanol.
(b) D
Key Concept
Reduction Reaction
Explanation
The reduction of disulfide bonds in the cyclic structure leads to the formation of thiol groups.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question