
Sia
Q1- Can you guess the type of chemical bond for the .red colored compound in the following equations
2- à Soluble
Q2- In an ionic solution, ammonium chloride was mixed with potassium phosphate
1- Write the molecular formula for the reactants
2- Do these solutions dissolve in water or not?
3- Write a balanced molecular equation to show the results
4- Based on the reaction results, is the reaction a precipitation reaction?
5 - Write a complete ionic equation
6 - Write a net ionic equation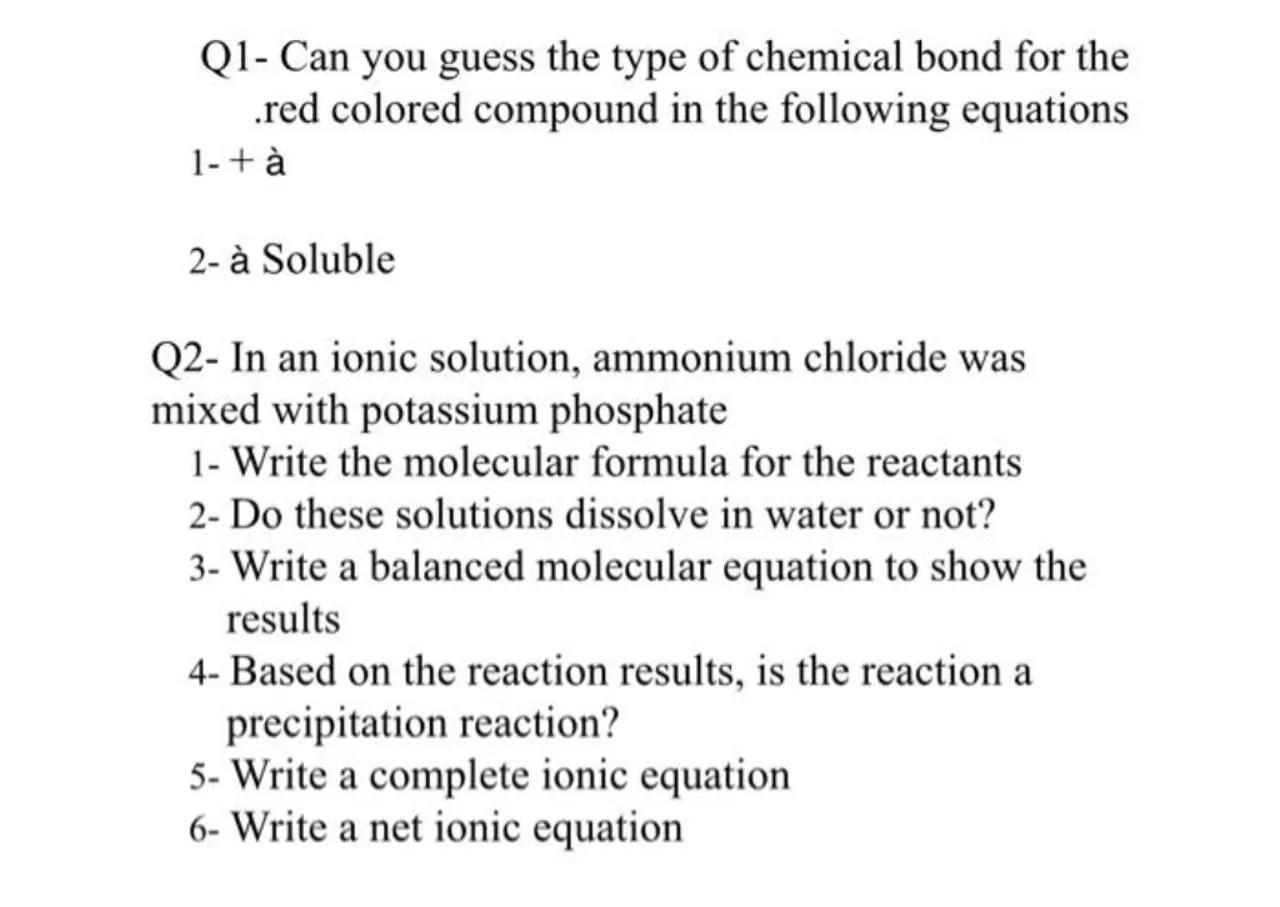

Answer from Sia
Posted over 1 year ago
Solution
1
Identify the red-colored compound: Without the specific chemical equations, it is not possible to determine the type of chemical bond in the red-colored compound. More information is needed to make an accurate guess
Q1 Answer
The type of chemical bond for the red-colored compound cannot be determined without additional information.
Key Concept
Chemical Bond Identification
Explanation
To identify the type of chemical bond, the specific compound or its chemical formula is required.
1
Write molecular formulas: The molecular formula for ammonium chloride is , and for potassium phosphate, it is
2
Determine solubility: Both and are soluble in water
3
Balanced molecular equation: The balanced molecular equation is NH_4Cl(aq) + K_3PO_4(aq) \rightarrow NH_4_3PO_4(aq) + 3KCl(aq)
4
Precipitation reaction: No solid precipitate forms in this reaction, so it is not a precipitation reaction
5
Complete ionic equation: The complete ionic equation is
6
Net ionic equation: Since all ions remain in solution and no precipitate is formed, the net ionic equation shows no reaction: all species are spectators
Q2 Answer
No precipitation reaction occurs when ammonium chloride is mixed with potassium phosphate in an ionic solution.
Key Concept
Precipitation Reactions and Ionic Equations
Explanation
A precipitation reaction occurs when two soluble salts react in solution to form an insoluble salt, which precipitates out of the solution. Since no precipitate forms in this reaction, the net ionic equation shows no change.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question