
Sia
Q5. In the 1H NMR of 1,3,4,6-tetra-O-acetyl-2-amino-2-deoxy- -Dglucopyranoside, the proton appears as a doublet (d) at ( ) while the proton appears as a doublet of doublet (dd) at with . From these data determine which of the following chair conformations or is adopted by the title compound and explain your answer.
The compound adopts the
conformation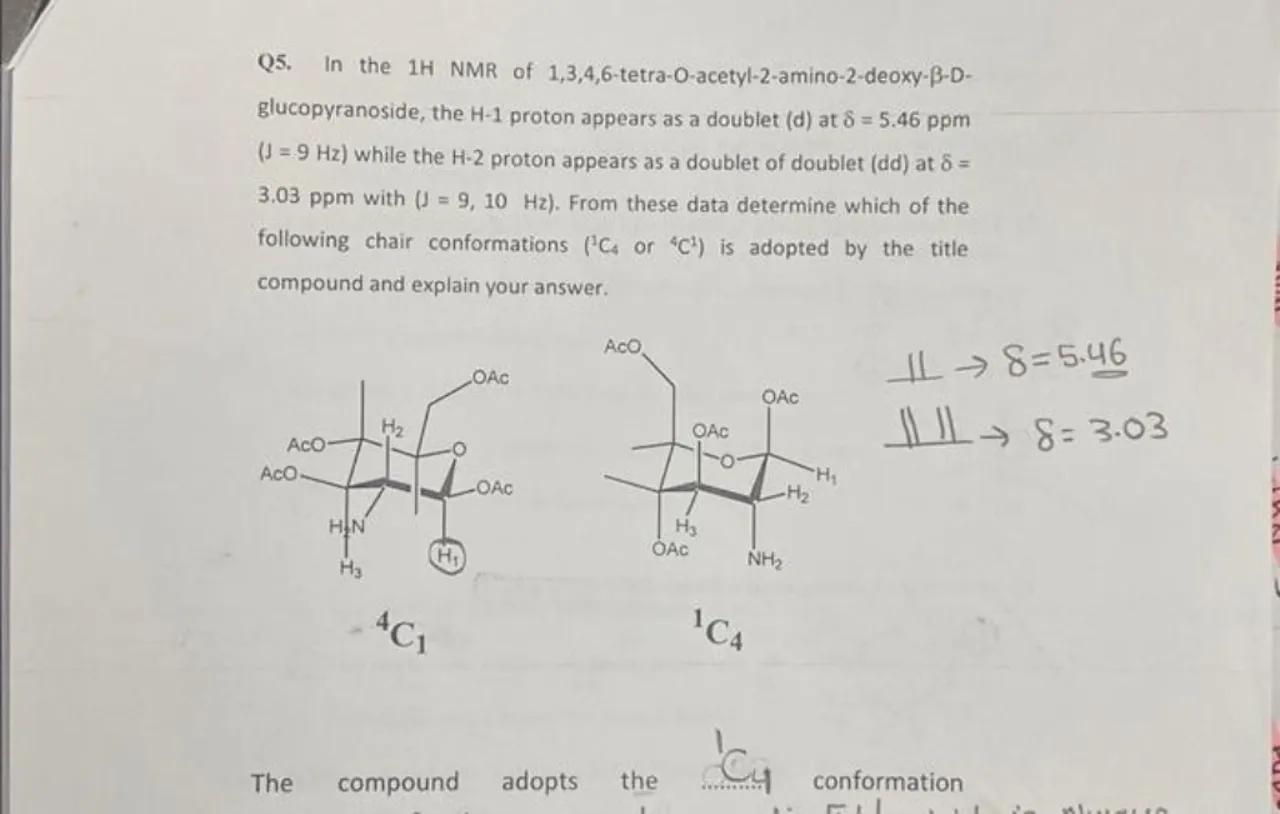

Answer from Sia
Posted almost 2 years ago
Solution
1
Analyze the NMR data for H-1: The H-1 proton appears as a doublet at δ = 5.46 ppm with a coupling constant (J) of 9 Hz. This suggests that H-1 is coupled to only one neighboring proton with a relatively large coupling constant, indicative of a dihedral angle of about 60° between the coupled protons, which is typical for an axial-axial coupling in a chair conformation
2
Analyze the NMR data for H-2: The H-2 proton appears as a doublet of doublets at δ = 3.03 ppm with coupling constants J = 9 Hz and J = 10 Hz. This indicates that H-2 is coupled to two different protons with similar coupling constants, suggesting that H-2 is in an equatorial position, as axial-equatorial couplings are typically smaller than axial-axial couplings
3
Determine the chair conformation: Considering the coupling constants and the chemical shifts, the data suggest that H-1 is axial (due to the larger coupling constant) and H-2 is equatorial (due to the presence of two coupling constants). The ^1C_4 conformation would place H-1 in an equatorial position and H-2 in an axial position, which is inconsistent with the NMR data. Therefore, the ^4C_1 conformation is adopted by the compound, as it places H-1 in an axial position and H-2 in an equatorial position, consistent with the observed NMR data
Answer
The compound adopts the ^4C_1 conformation.
Key Concept
Coupling constants in NMR spectroscopy can help determine the spatial arrangement of protons in a molecule.
Explanation
The larger coupling constant for H-1 suggests an axial position, while the doublet of doublets for H-2 suggests an equatorial position. The ^4C_1 conformation is consistent with these observations.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question
