
Sia

Question
Chemistry
Posted almost 2 years ago
Question 2 (4 points)
For the following reaction, pick out the major product: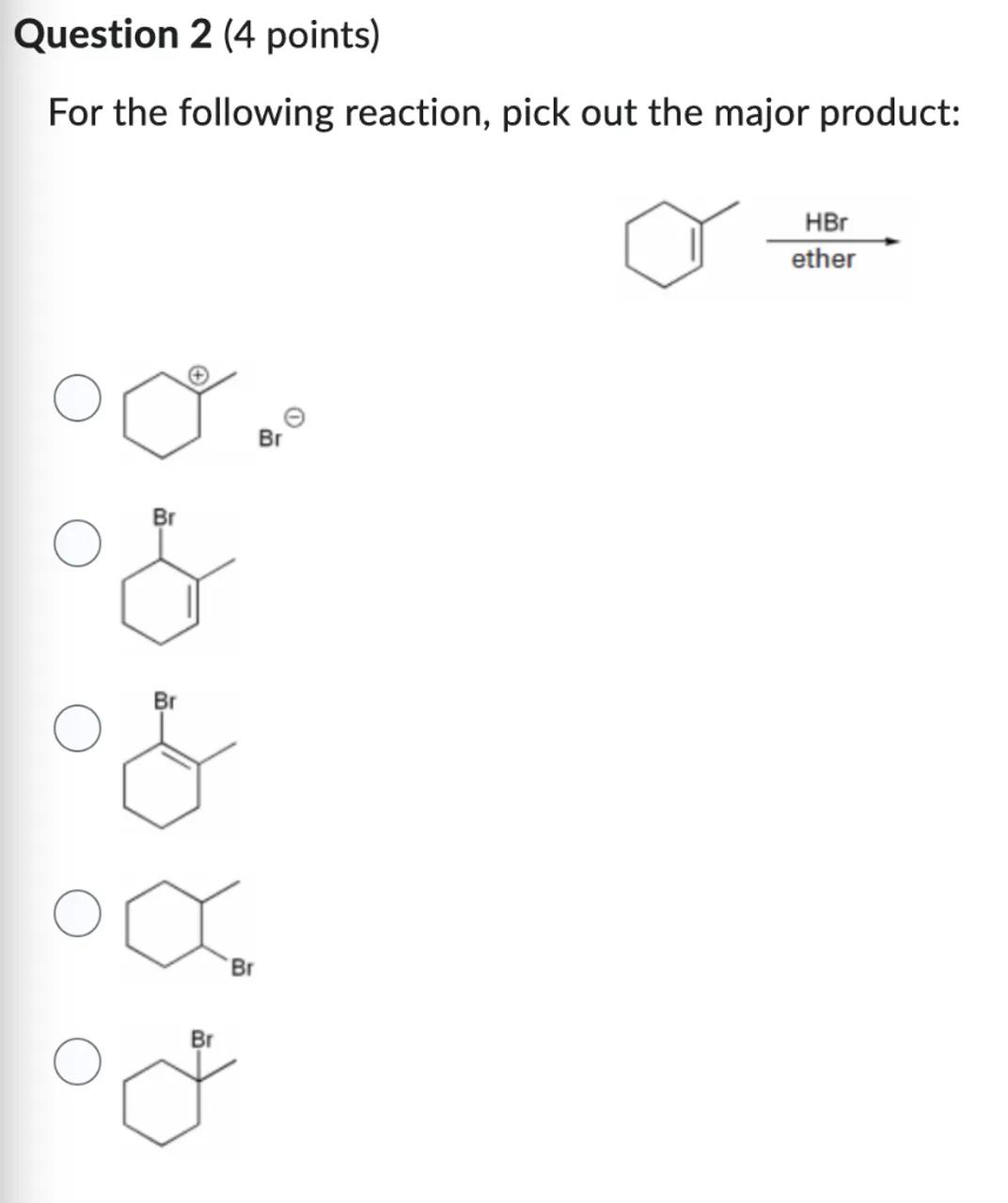

Answer from Sia
Posted almost 2 years ago
B
Key Concept
Markovnikov's rule and carbocation stability
Explanation
In the addition of HBr to 1-methylcyclohexene, the major product is formed according to Markovnikov's rule, which states that the hydrogen atom will add to the carbon with the greater number of hydrogen atoms (the less substituted carbon). This leads to the formation of the more stable tertiary carbocation intermediate, which then reacts with the bromide ion to give the major product. The product with the bromine atom at the tertiary carbon (the more substituted carbon) is favored.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question