
Sia
Question 5: Conformations (5 Marks): The elimination shown below has a very large difference in the rate of reaction that depends on the stereochemistry of the substrate. Please use chair conformations in the space below to explain the results shown in the box. Please provide a brief explanation of the observed reactivity difference in the second box.
Chair conformations:(4 marks)
Explanation: (1 mark)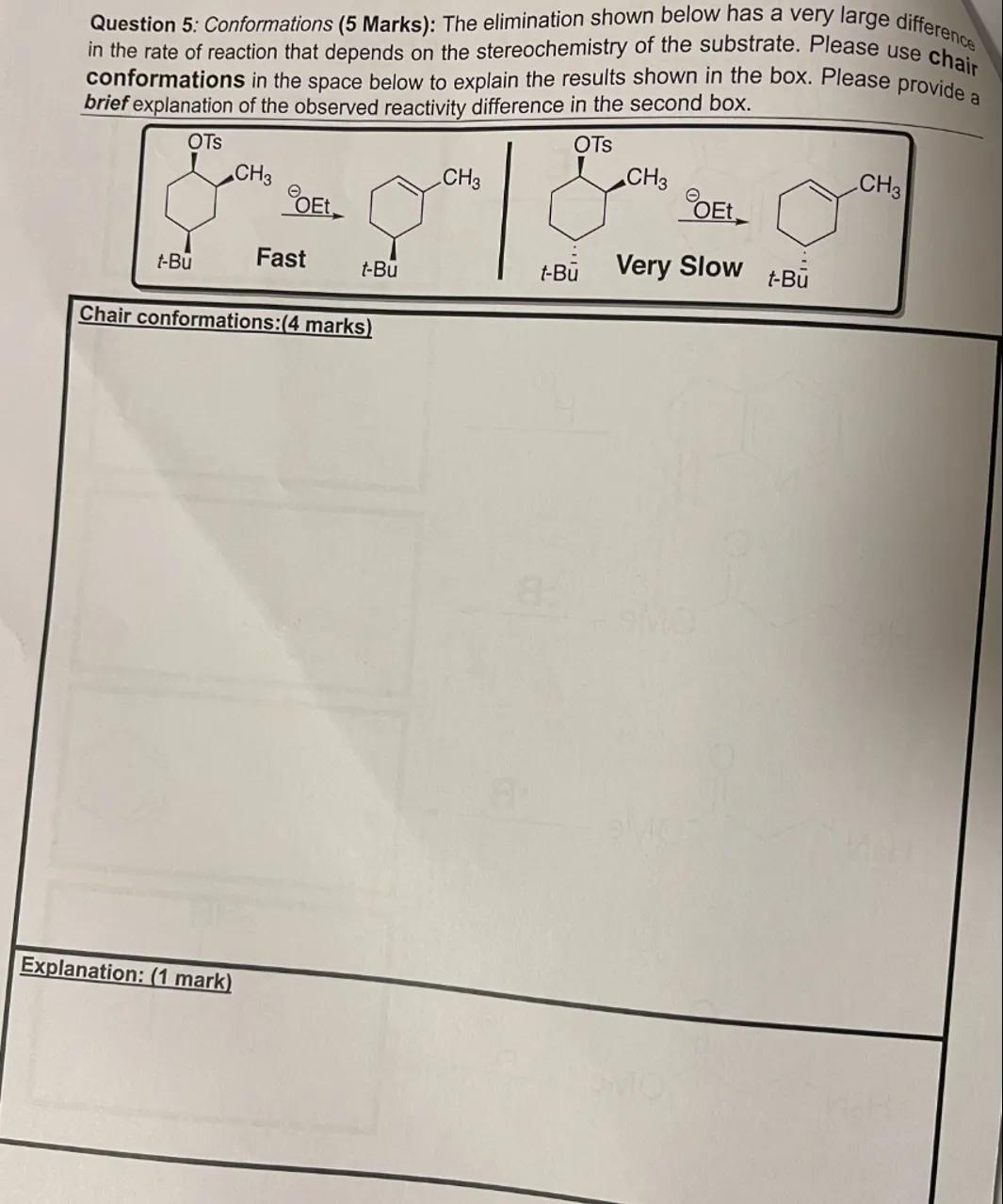

Answer from Sia
Posted almost 2 years ago
Solution
1
Draw the chair conformations: To explain the difference in reaction rates, we must consider the stability and steric hindrance in the chair conformations of the cyclohexane ring. In the 'Fast' conformation, the t-Bu group is equatorial, minimizing steric hindrance, while the OEt group is axial, positioned ideally for an E2 elimination reaction. In the 'Very Slow' conformation, the t-Bu group is axial, causing significant steric hindrance and destabilizing the conformation, making the E2 reaction much less favorable
2
Explain the observed reactivity difference: The rate of an E2 elimination reaction is highly dependent on the steric environment around the leaving group. In the 'Fast' conformation, the ethoxy group (OEt) is anti-periplanar to the hydrogen that will be abstracted by the base, facilitating the E2 mechanism. In the 'Very Slow' conformation, the bulky t-Bu group is in an axial position, which increases steric hindrance and makes it difficult for the base to approach and abstract the hydrogen, thus slowing down the reaction
Answer
The difference in reaction rates is due to the steric hindrance and the orientation of the groups in the chair conformations. The 'Fast' conformation allows for an optimal E2 elimination, while the 'Very Slow' conformation is hindered by the axial t-Bu group.
Key Concept
Steric hindrance in chair conformations affects E2 elimination reaction rates.
Explanation
In the 'Fast' conformation, minimal steric hindrance allows for the E2 reaction to proceed rapidly, whereas in the 'Very Slow' conformation, significant steric hindrance from the axial t-Bu group slows the reaction.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question