
Sia
Question 8.
Nitric oxide reacts with oxygen to produce nitrogen dioxide according to the following reaction:
Here is the fourth digit of your URN number.
a) Calculate the equilibrium concentrations of all species in the mixture at from the initial concentration of and .
b) Calculate the equilibrium constant .
c) Assuming that the forward reaction is exothermic, comment on whether the forward or reverse reaction is favoured if the pressure, temperature, and volume of the reaction are increased.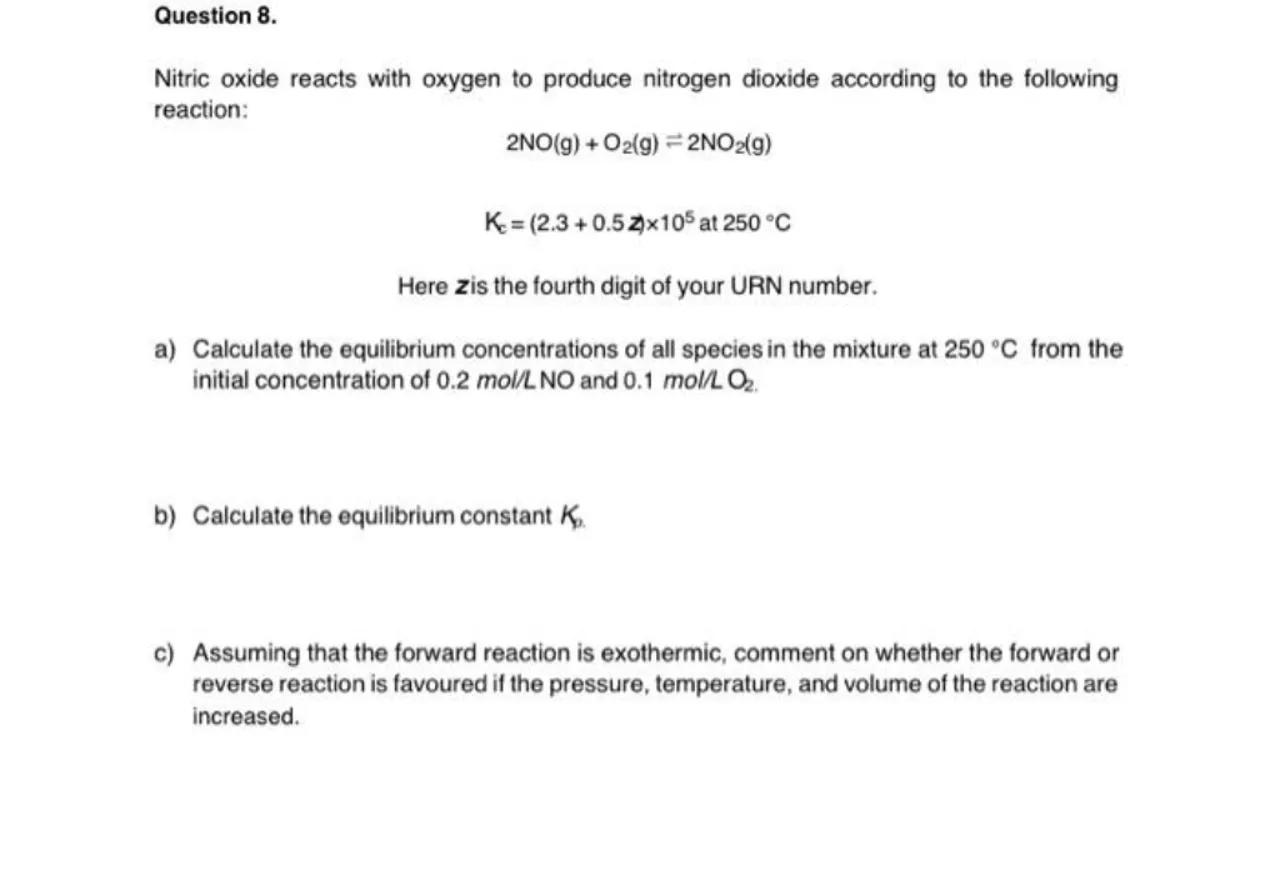

Answer from Sia
Posted almost 2 years ago
Solution
1
Set up the ICE table: To find the equilibrium concentrations, we use an ICE (Initial, Change, Equilibrium) table
2
Write the initial concentrations: NO = 0.2 M, O2 = 0.1 M, NO2 = 0 M
3
Express the changes in concentration: Let x be the change in concentration of NO and NO2. Since the stoichiometry is 2:1:2, the change for O2 will be x/2 and for NO2 will be x
4
Write the equilibrium concentrations: NO = 0.2 - 2x, O2 = 0.1 - x/2, NO2 = 0 + x
5
Apply the equilibrium constant expression: . Substitute the equilibrium concentrations and solve for x
6
Calculate x: Since we don't have the value of Z, we cannot find the exact value of x. However, the process would involve substituting the equilibrium concentrations into the expression and solving the resulting quadratic equation for x
a Answer
The equilibrium concentrations cannot be calculated without the value of Z.
Key Concept
The equilibrium concentrations of reactants and products are determined using an ICE table and the equilibrium constant expression.
Explanation
Without the value of Z, we cannot calculate the exact equilibrium concentrations, as Z is required to determine the value of the equilibrium constant, Kc.
Solution
1
Understand the relationship between Kc and Kp: The equilibrium constant Kp is related to Kc by the equation , where is the change in moles of gas and R is the ideal gas constant
2
Calculate Kp: Since for this reaction (2 moles of gas react to form 2 moles of gas),
b Answer
Kp = Kc =
Key Concept
Kp is related to Kc by the equation , but if the number of moles of gas does not change, Kp equals Kc.
Explanation
Since there is no change in the number of moles of gas in this reaction, Kp is equal to Kc, and we do not need to adjust for temperature or the ideal gas constant.
Solution
1
Understand Le Chatelier's Principle: Le Chatelier's Principle states that if a dynamic equilibrium is disturbed, the system will adjust to minimize the disturbance
2
Analyze the effect of pressure: Increasing pressure favors the side with fewer moles of gas. In this case, the number of moles is the same on both sides, so pressure has no effect
3
Analyze the effect of temperature: Increasing temperature favors the endothermic reaction. Since the forward reaction is exothermic, increasing temperature favors the reverse reaction
4
Analyze the effect of volume: Increasing volume decreases pressure, which favors the side with more moles of gas. Since the number of moles is the same on both sides, volume has no effect
c Answer
Increasing temperature favors the reverse reaction; pressure and volume changes do not affect the equilibrium position.
Key Concept
Le Chatelier's Principle predicts the direction of the shift in equilibrium when a system is disturbed.
Explanation
Since the forward reaction is exothermic, increasing temperature will favor the reverse reaction. Changes in pressure and volume do not affect the equilibrium because the number of moles of gas is the same on both sides of the reaction.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question