
Sia
Scoring: Your score wili penalty for missing matches.
Use the References to access important values if needed for th
Arrange the following compounds in order of increasing boiling point.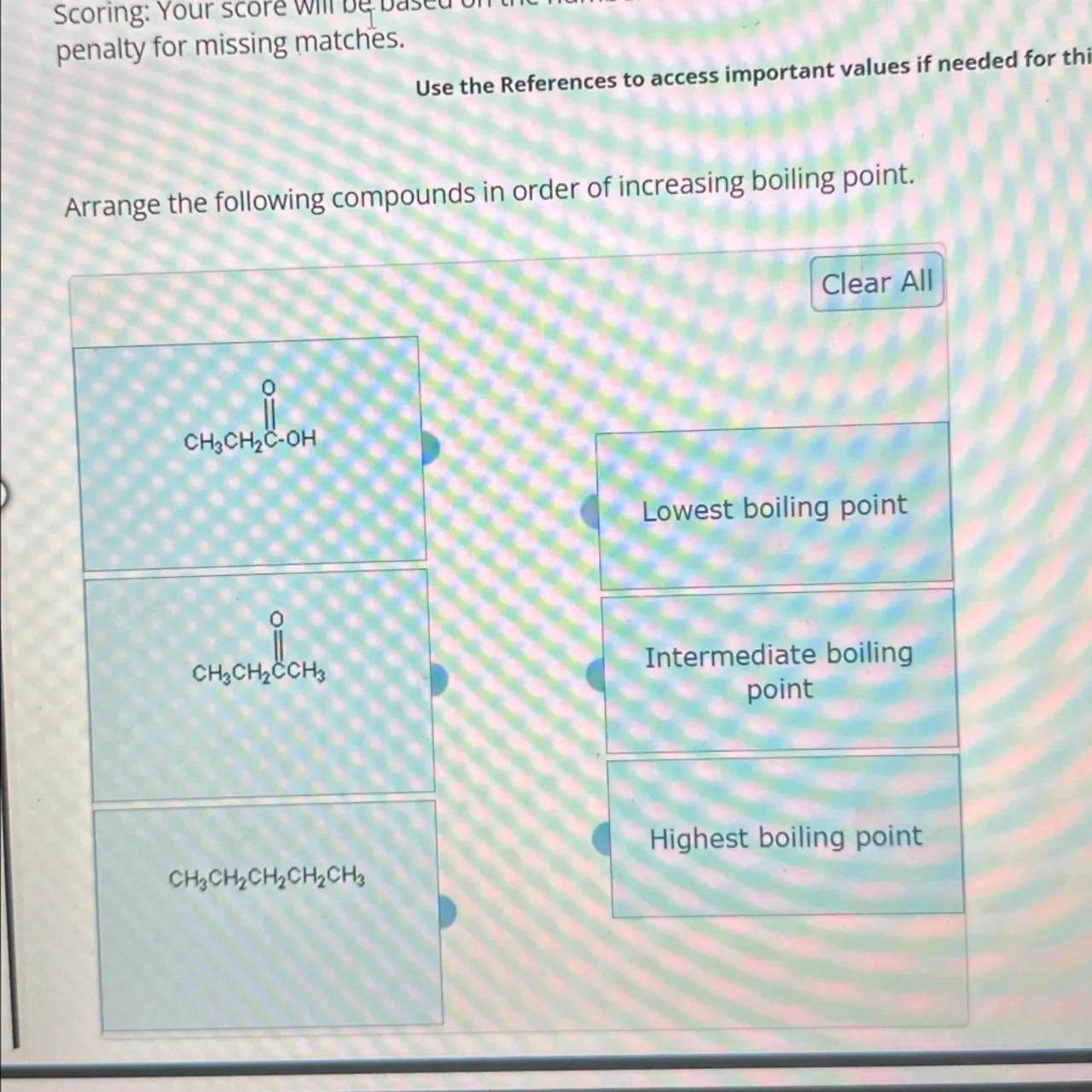

Answer from Sia
Posted almost 2 years ago
Solution
1
Identify the compounds: The three organic compounds given are propanal (CH3CH2COH), acetone (CH3CH2COCH3), and butane (CH3CH2CH2CH3)
2
Analyze intermolecular forces: The boiling point of a compound is largely determined by the strength of its intermolecular forces. Propanal has hydrogen bonding due to the presence of an aldehyde group, acetone has dipole-dipole interactions due to the presence of a ketone group, and butane has only London dispersion forces as it is a nonpolar molecule
3
Rank by boiling point: Compounds with hydrogen bonding typically have the highest boiling points, followed by those with dipole-dipole interactions, and then those with London dispersion forces
4
Arrange the compounds: Based on the strength of intermolecular forces, the compounds can be arranged in order of increasing boiling point: butane (lowest boiling point), acetone (intermediate boiling point), and propanal (highest boiling point)
Answer
CH3CH2CH2CH3 < CH3CH2COCH3 < CH3CH2COH
Key Concept
Intermolecular forces determine boiling points
Explanation
The strength of intermolecular forces (hydrogen bonding > dipole-dipole > London dispersion) dictates the order of increasing boiling points for the given compounds.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question