
Sia
Select the appropriate atoms by clicking them so that they are highlighted.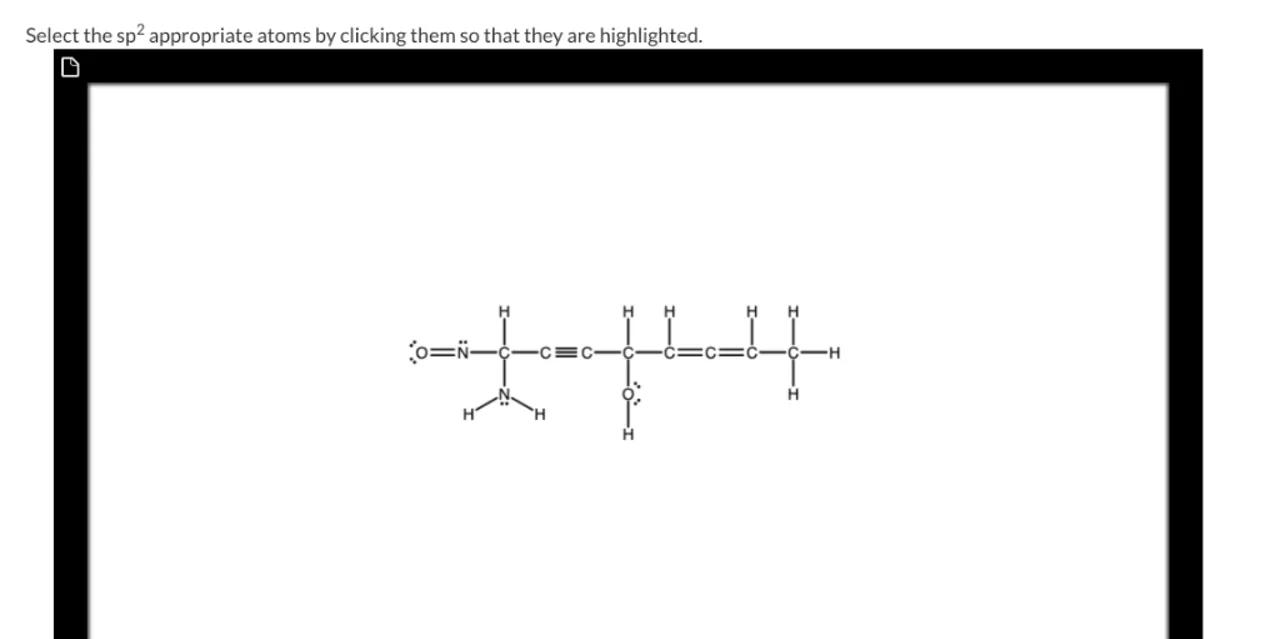

Answer from Sia
Posted almost 2 years ago
Solution
1
Identify the $sp^2$ hybridized atoms: In the given skeletal structure, hybridized atoms are typically found in double bonds or in atoms with three regions of electron density. These atoms have a trigonal planar geometry
2
Locate the double bonds: Look for carbon atoms that are part of double bonds. These carbon atoms are hybridized
3
Check for other $sp^2$ hybridized atoms: Besides carbon, other atoms like oxygen and nitrogen can also be hybridized if they are part of a double bond or have a lone pair and a double bond
4
Highlight the $sp^2$ hybridized atoms: Click on the atoms identified in the previous steps to highlight them
Answer
The hybridized atoms are those involved in double bonds or with three regions of electron density.
Key Concept
hybridization occurs in atoms with three regions of electron density, typically found in double bonds.
Explanation
Atoms with hybridization have a trigonal planar geometry and are commonly found in double bonds or atoms with a lone pair and a double bond.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question