
Sia

Question
Chemistry
Posted over 1 year ago
Steam is compressed from and to isentropically. Determine the final temperature of the steam.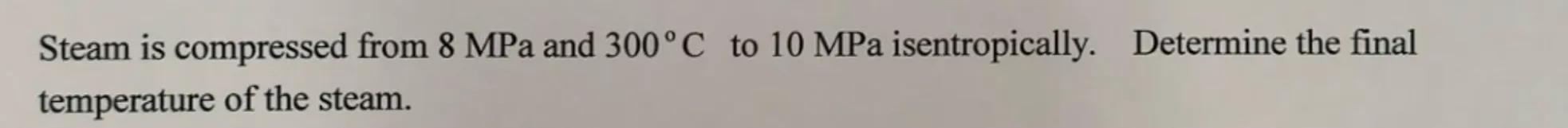

Answer from Sia
Posted over 1 year ago
Solution
1
Identify the initial and final states: The initial state of the steam is at and . The final state is at , and the process is isentropic
2
Use steam tables or Mollier diagram: To find the final temperature, we need to use steam tables or a Mollier diagram (enthalpy-entropy chart) for water
3
Determine initial entropy: From the steam tables, find the specific entropy () at and
4
Find final temperature: Using the same specific entropy value, find the corresponding temperature at from the steam tables or Mollier diagram
Answer
The final temperature of the steam after isentropic compression from and to is approximately .
Key Concept
Isentropic process
Explanation
In an isentropic process, entropy remains constant. By using steam tables or Mollier diagrams, we can find the final temperature corresponding to the same entropy at the new pressure.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question