
Sia

Question
Chemistry
Posted almost 2 years ago
Suppose a student performed an experiment similar to Experiment 1 and collected the data shown in the table.
\begin{tabular}{|c|c|}
\hline mass of cold water in the calorimeter & \\
\hline initial temperature of the cold water & \\
\hline mass of hot water added to the calorimeter & \\
\hline initaal tengerature of the hot water & \\
\hline flal temperature in the caborimeter & \\
\hline
\end{tabular}
Calculate the calorimeter constant for the calorimeter the student used in the experiment.
calorimeter constant: I/C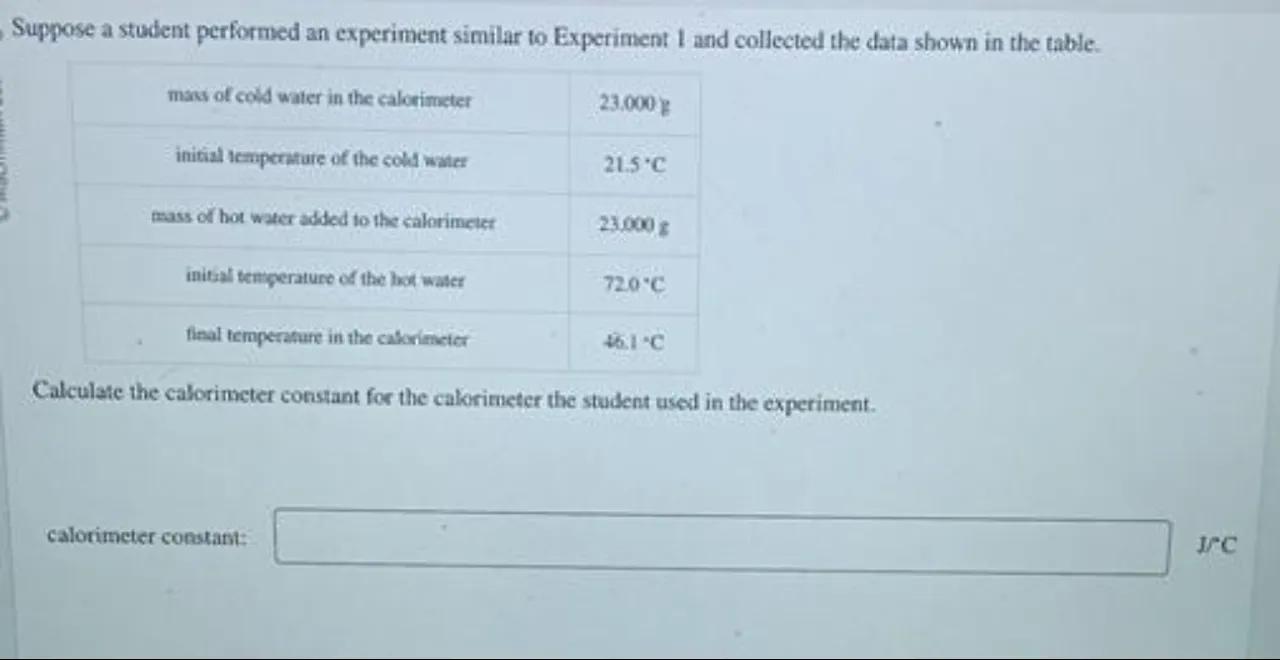

Answer from Sia
Posted almost 2 years ago
Solution
1
Identify the data from the table: The data provided in the table includes the mass of cold water (), the initial temperature of cold water (), the mass of hot water (), the initial temperature of hot water (), and the final temperature in the calorimeter ()
2
Apply the principle of calorimetry: The heat lost by the hot water will be equal to the heat gained by the cold water and the calorimeter. This can be expressed as , where is the specific heat capacity of water (4.184 J/g°C) and is the calorimeter constant we need to find
3
Calculate the calorimeter constant: Rearrange the equation to solve for : . Plug in the values from the table and calculate
Answer
The calorimeter constant cannot be calculated with the given data because the final temperature is lower than the initial temperature of the cold water, which is physically impossible in this context. There may be a typo in the provided temperatures.
Key Concept
The calorimeter constant is determined by the heat exchange between the hot and cold water and the calorimeter itself.
Explanation
The calorimeter constant is calculated based on the principle that the heat lost by the hot water is equal to the heat gained by the cold water and the calorimeter. However, the provided data contains inconsistencies that make the calculation impossible.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question