
Sia
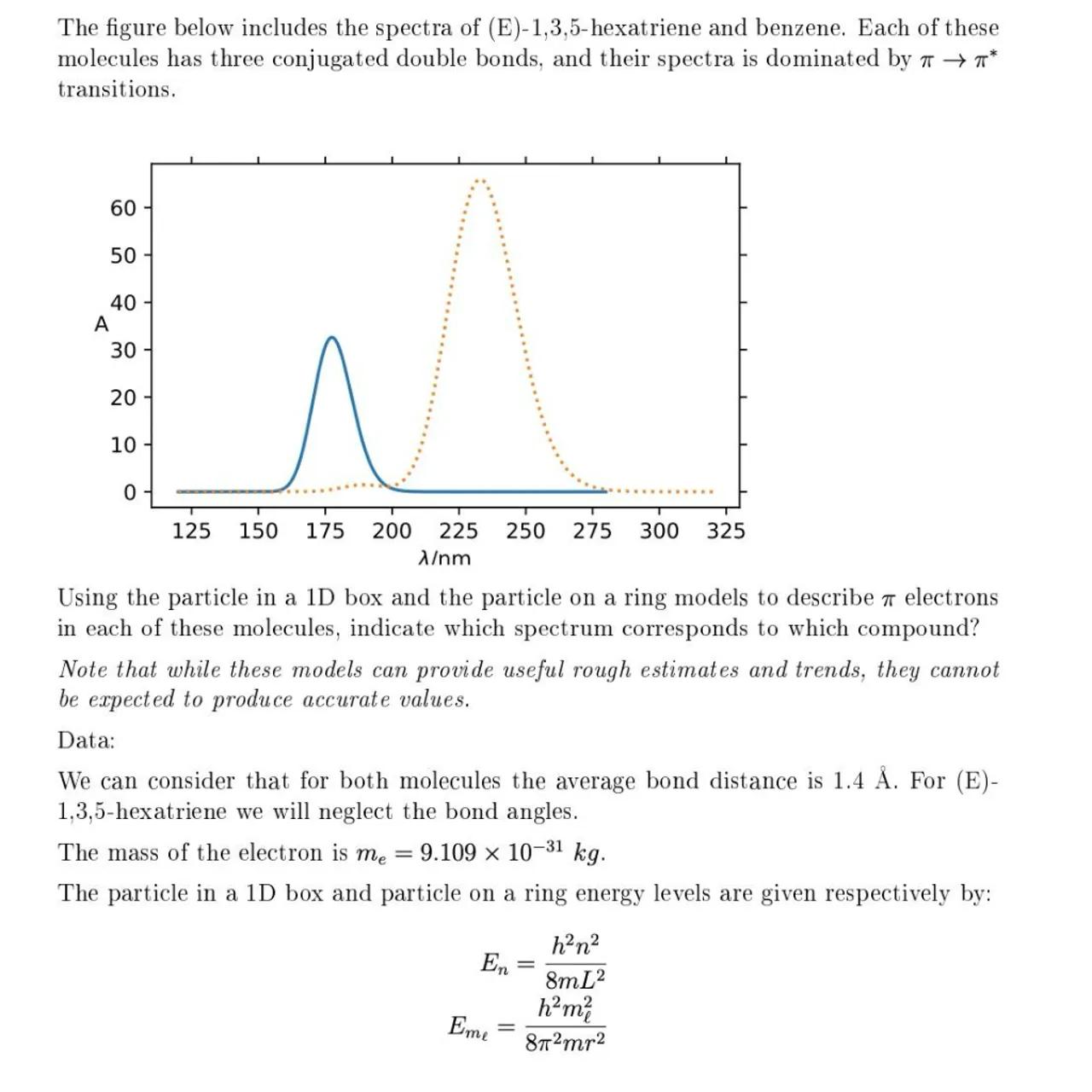
The figure below includes the spectra of (E)-1,3,5-hexatriene and benzene. Each of these molecules has three conjugated double bonds, and their spectra is dominated by transitions.
Using the particle in a 1D box and the particle on a ring models to describe electrons in each of these molecules, indicate which spectrum corresponds to which compound?
Note that while these models can provide useful rough estimates and trends, they cannot be expected to produce accurate values.
Data:
We can consider that for both molecules the average bond distance is 1.4 \AA. For (E)-hexatriene we will neglect the bond angles.
The mass of the electron is .
The particle in a 1D box and particle on a ring energy levels are given respectively by:

Answer from Sia
Posted almost 2 years ago
Solution
1
Determine the length of the box for (E)-1,3,5-hexatriene: For (E)-1,3,5-hexatriene, which can be modeled as a particle in a 1D box, the length of the box () is the length of the conjugated system. Since we are neglecting bond angles, we can simply add the bond distances together. There are 4 bonds in the conjugated system, each with a length of
2
Calculate the length of the box:
3
Determine the circumference of the ring for benzene: For benzene, modeled as a particle on a ring, the circumference () is the length of the conjugated system. Benzene has 6 carbon atoms, with each C-C bond considered to be
4
Calculate the circumference of the ring: ; thus,
5
Relate the energy levels to the wavelength of light absorbed: The energy difference between the ground state and the first excited state corresponds to the wavelength of light absorbed. For the particle in a 1D box, this is between and , and for the particle on a ring, it is between and
6
Calculate the energy difference for (E)-1,3,5-hexatriene: Using the formula for the particle in a 1D box, , we find the energy difference
7
Calculate the energy difference for benzene: Using the formula for the particle on a ring, , we find the energy difference
8
Convert the energy difference to wavelength: The wavelength of light absorbed () is related to the energy difference by , where is Planck's constant, is the speed of light, and is the energy difference
9
Compare the calculated wavelengths with the given spectra: The molecule with the larger box or ring circumference will have a smaller energy difference and thus absorb light at a longer wavelength
10
Identify the spectra: Since benzene has a larger circumference than (E)-1,3,5-hexatriene, it will absorb at a longer wavelength. Therefore, the spectrum with the peak at 250 nm corresponds to benzene, and the one with the peak at 175 nm corresponds to (E)-1,3,5-hexatriene
Answer
The spectrum with the peak at 175 nm corresponds to (E)-1,3,5-hexatriene, and the spectrum with the peak at 250 nm corresponds to benzene.
Key Concept
The particle in a 1D box and the particle on a ring models are used to estimate the wavelengths of light absorbed by conjugated systems based on their geometry.
Explanation
The length of the conjugated system affects the energy levels of the electrons. A longer system, such as benzene's ring, results in closer energy levels and absorption at a longer wavelength compared to a shorter system like (E)-1,3,5-hexatriene.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question
