
Sia
V. Determine the structure of each of the following compounds
a)
2
3
3
\begin{tabular}{llllllllllllllllll}
\hline 1 & 1 & 1 & 1 & 1 & 1 & 1 & 1 & 1 & 1 & 1 \\
8 & 7 & 6 & 5 & 4 & 3 & 2 & 1 & 0
\end{tabular}
ppm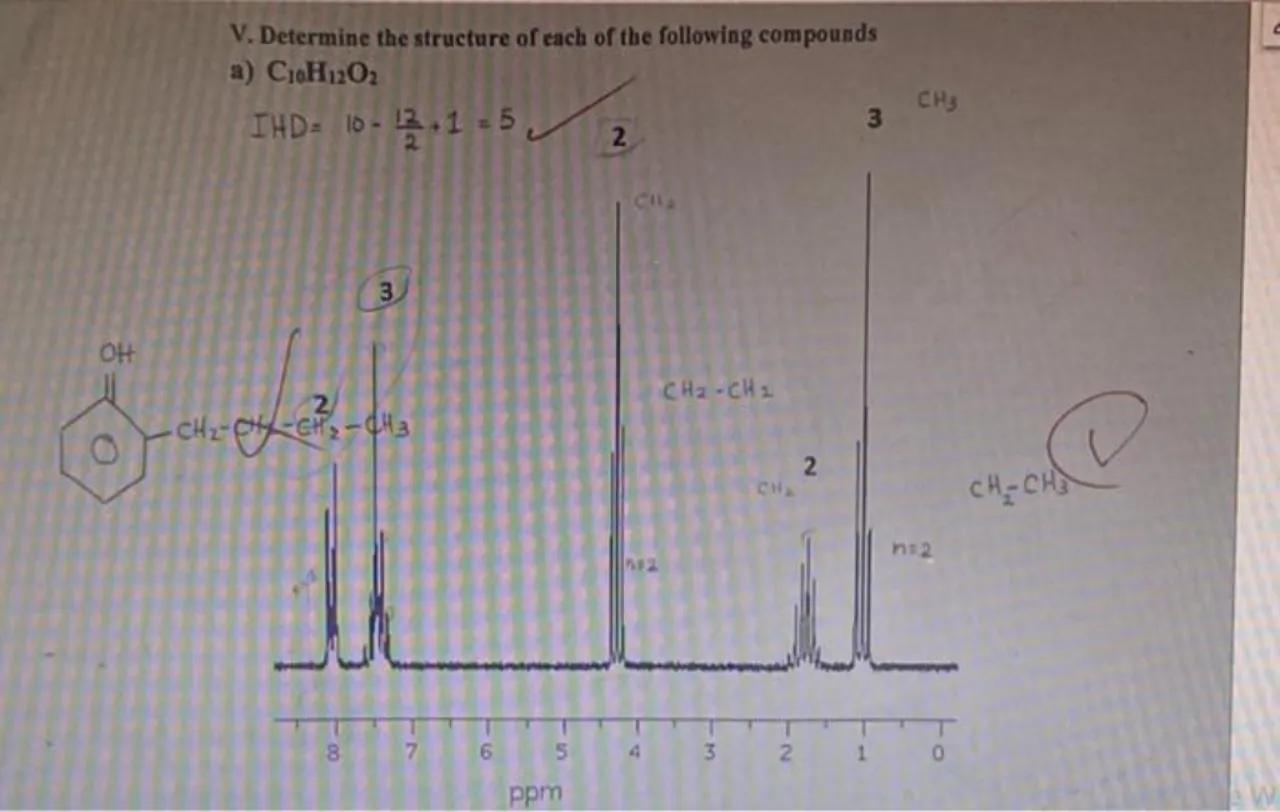

Answer from Sia
Posted almost 2 years ago
Solution
1
Calculate the Index of Hydrogen Deficiency (IHD): The IHD is used to determine the number of rings and/or multiple bonds in a molecule. It is calculated using the formula , where C is the number of carbons, H is the number of hydrogens, X is the number of halogens, and N is the number of nitrogens. For the given compound , there are no halogens or nitrogens, so the formula simplifies to . Plugging in the values, we get
2
Analyze the NMR spectrum: The NMR spectrum provides information about the different hydrogen environments in the molecule. The peak around 0.9 ppm labeled as "CH3" suggests the presence of methyl groups. The peak around 1.2 ppm labeled as "CH2-CH2" indicates the presence of methylene groups adjacent to each other. The "n+2" annotation suggests the multiplicity of the signals, which helps in identifying the number of neighboring hydrogens
3
Determine the structure: Based on the IHD of 5, we know there are five elements of unsaturation, which could be a combination of double bonds and rings. The presence of a benzene ring accounts for 4 elements of unsaturation (1 ring + 3 double bonds). The remaining IHD of 1 suggests another double bond or ring. The chemical shifts in the NMR spectrum indicate the presence of methyl and methylene groups, which helps in constructing the rest of the molecule
Answer
The structure of the compound with an IHD of 5 is likely to be a benzene ring with a hydroxyl group and a side chain containing a double bond or an additional ring. The NMR spectrum suggests the presence of methyl and methylene groups, which are part of the side chain.
Key Concept
Index of Hydrogen Deficiency (IHD)
Explanation
IHD is used to determine the number of rings and/or multiple bonds in a molecule, which is crucial for deducing the structure of an organic compound.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question
