
Sia
Which of the following sets of systems are in order of increasing entropy?
(A)
(B)
(C)
(D) 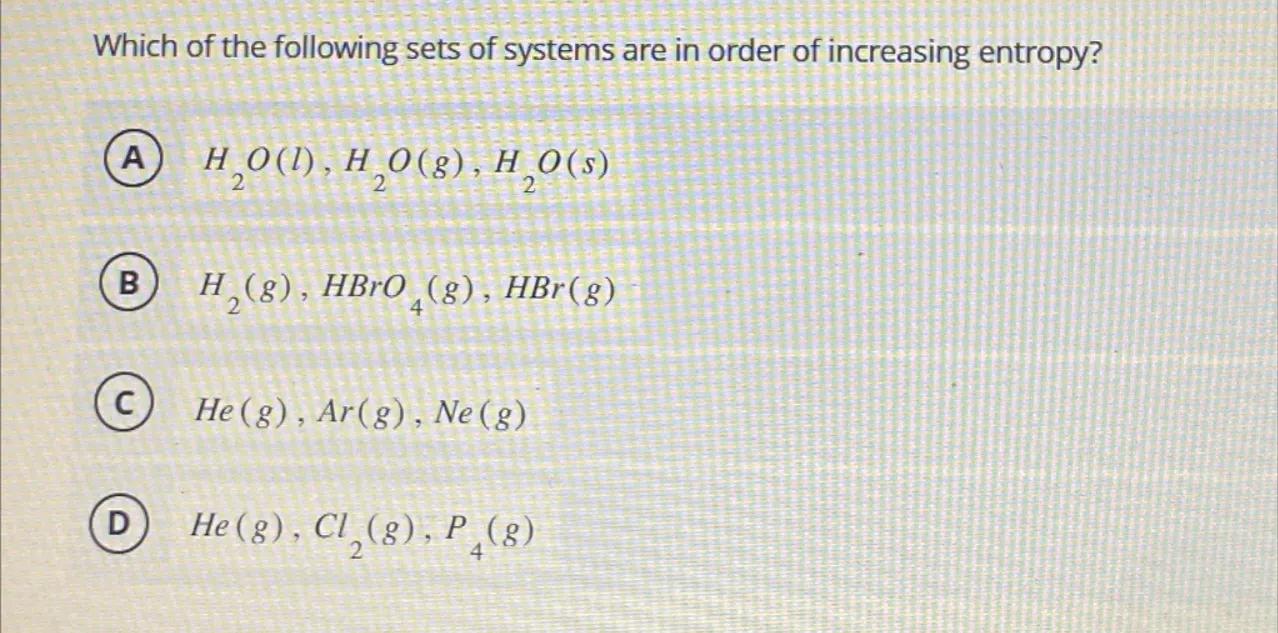

Answer from Sia
Posted almost 2 years ago
D
Key Concept
Entropy
Explanation
Entropy is a measure of disorder or randomness in a system. In gases, entropy is higher due to the greater freedom of movement of the particles. Among the given options, , , and are in order of increasing molecular complexity and thus increasing entropy.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question