
Sia

Question
Chemistry
Posted almost 2 years ago
Write the mechanism of free radical polymerisation of the following reaction:
Initiator: AIBN
Monomer: methyl methacrylate
The mechanism should include:
1. Initiation of primary radical and activated monomer
2. Propagation - head-to-tail addition
3. Termination-combination and disproportination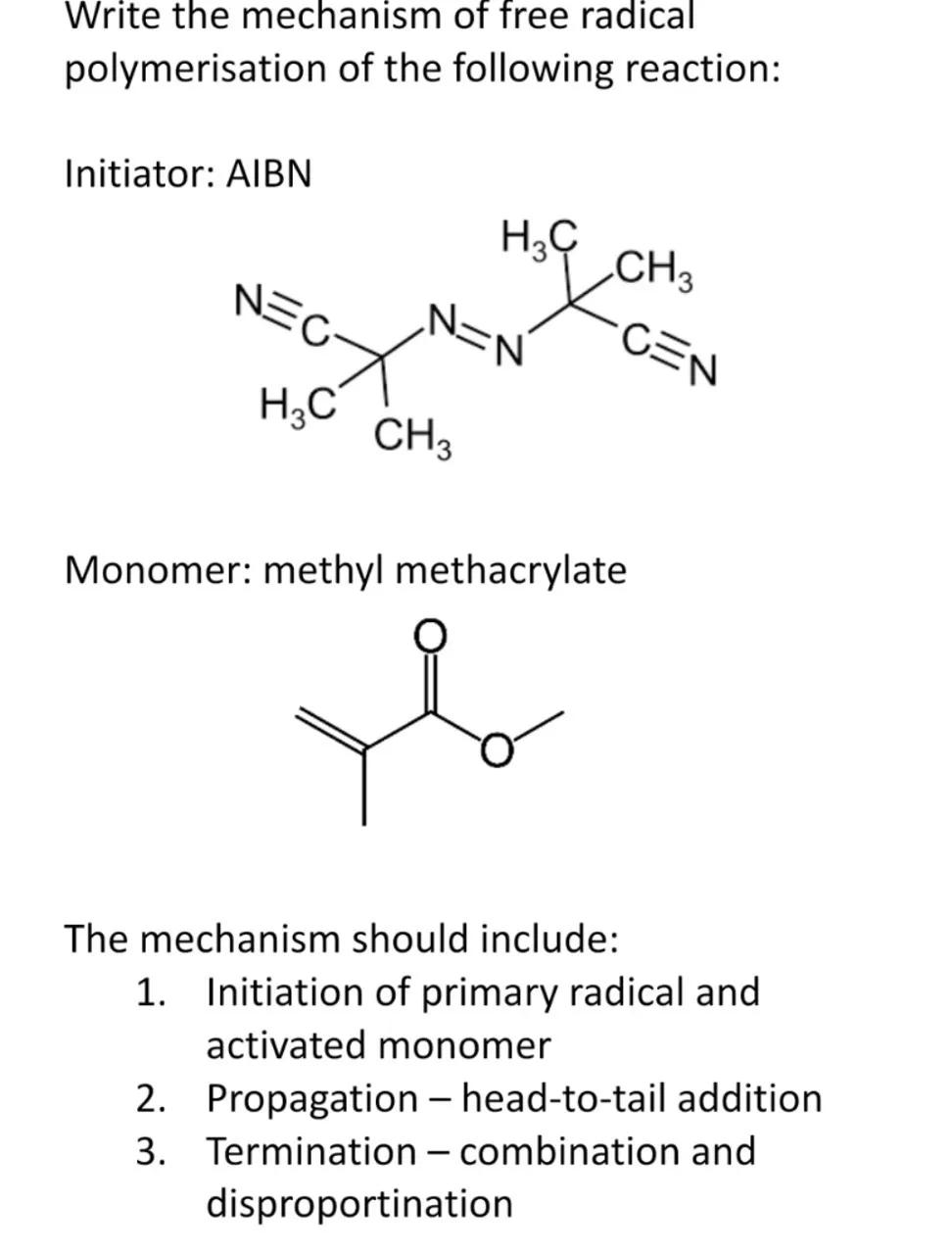

Answer from Sia
Posted almost 2 years ago
Solution
1
Initiation: The initiation step involves the thermal decomposition of AIBN to form two cyanopropyl radicals. AIBN decomposes into two radicals when heated, each radical having the structure C(CH)CN. These radicals are very reactive and can initiate the polymerization process by reacting with a monomer molecule
2
Propagation: During propagation, the radical adds to the double bond of a methyl methacrylate monomer, forming a new radical at the end of the growing polymer chain. This step is repeated as the radical at the end of the chain reacts with additional monomer molecules, adding them to the growing chain in a head-to-tail fashion. The general form of the propagation step can be represented as , where is the growing polymer chain with monomer units and is a monomer molecule
3
Termination: Termination can occur by combination, where two growing radical chains join together to form a single, non-radical polymer, or by disproportionation, where hydrogen is transferred from one radical to another, creating a saturated end on one polymer chain and a double bond at the end of the other
Answer
The mechanism of free radical polymerization of methyl methacrylate initiated by AIBN includes initiation, propagation, and termination steps.
Key Concept
Free radical polymerization involves initiation, propagation, and termination steps.
Explanation
Initiation starts with the formation of radicals from the initiator, propagation involves the addition of monomers to the growing chain, and termination ends the reaction by combining or disproportionating the active chain ends.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question