
Sia

Question
Chemistry
Posted almost 2 years ago
\begin{tabular}{|c|c|}
\hline \multicolumn{2}{|c|}{ [Substane| } \\
\hline C(s graphite) & 5.7 \\
\hline C(s diamond & d) 2.4 \\
\hline & 114.7 \\
\hline & 130.7 \\
\hline & 165.2 \\
\hline & 223.1 \\
\hline & 234.6 \\
\hline
\end{tabular}
Part A
Find the deltaSdegree for the formation of from its elements in their standard states
deltaSrxn 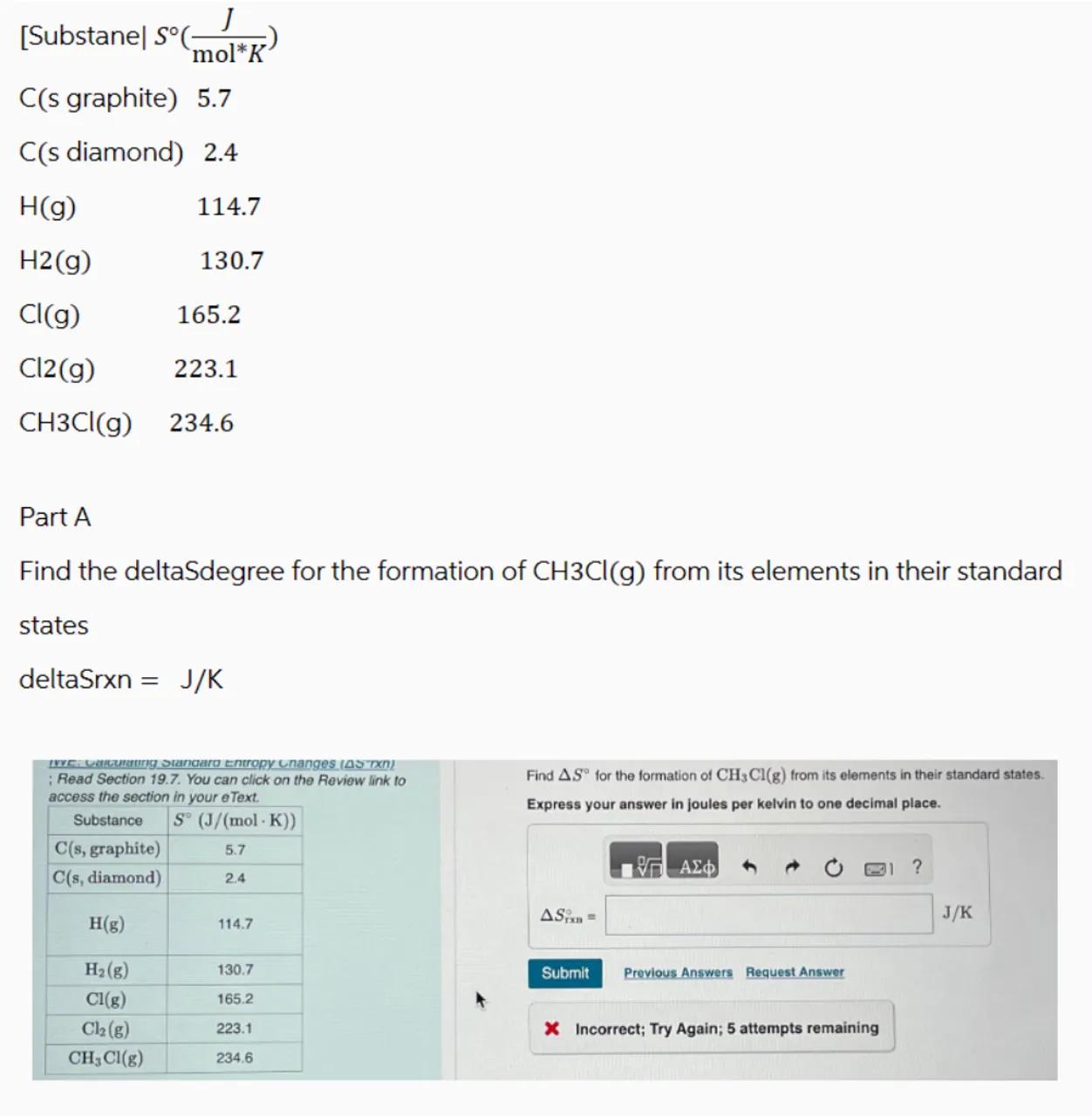

Answer from Sia
Posted almost 2 years ago
Solution
1
Write the balanced chemical equation for the formation of CH3Cl(g): The formation of CH3Cl(g) from its elements in their standard states can be represented by the following equation:
2
Calculate the change in standard entropy (ΔS°): The change in standard entropy for the reaction (ΔS°rxn) is calculated using the standard molar entropies (S°) of the reactants and products. The formula to calculate ΔS°rxn is: . Using the values from the table: . Plugging in the values:
3
Perform the calculation: Now we perform the arithmetic:
Answer
-274.8 J/(mol*K)
Key Concept
Entropy change for a reaction
Explanation
The entropy change for a reaction is calculated by subtracting the sum of the standard molar entropies of the reactants from the sum of the standard molar entropies of the products.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question