
Sia
\begin{tabular}{|l|l|l|}
\hline Trial & {} & Absorbance \\
\hline 1 & 0.025 & 0.124 \\
\hline 2 & 0.050 & 0.268 \\
\hline 3 & 0.100 & 0.520 \\
\hline 4 & 0.150 & 0.680 \\
\hline
\end{tabular}
Using a spectrophotometer, a student measures the absorbance of four solutions of at a given wavelength. The collected data is given in the table above. Which of the following is the most likely explanation for the discrepant data in trial 4 ?
(A) The solution was at a lower temperature than the solutions in the other trials.
(B) The measurement was made using a different spectrophotometer that uses a cell with a longer path length.
C) The solution was saturated and the flow of light through the solution was restricted.
(D) The concentration of the solution was actually lower than .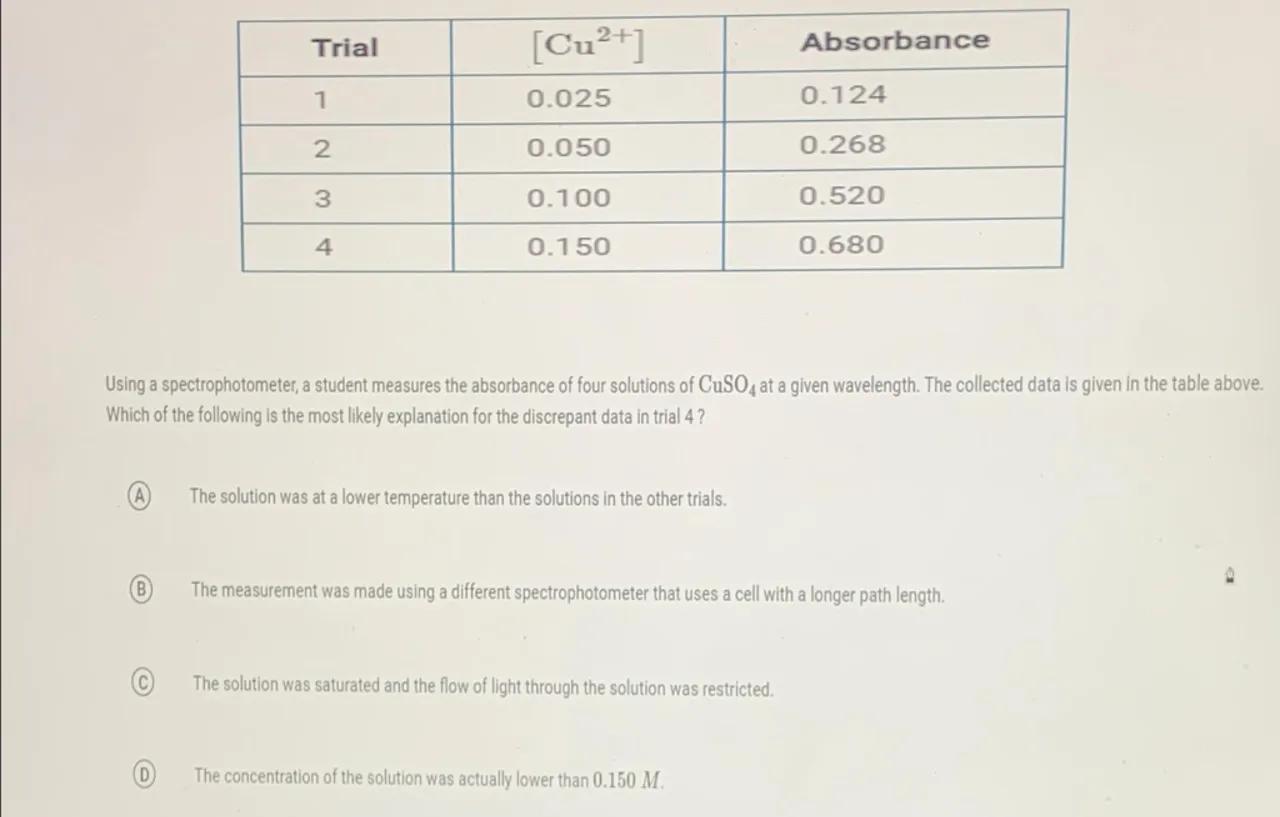

Answer from Sia
Posted almost 2 years ago
B
Key Concept
Path length in spectrophotometry
Explanation
The absorbance of a solution in spectrophotometry is directly proportional to the path length of the cell used. If a different spectrophotometer with a longer path length was used for Trial 4, it would result in a higher absorbance reading, explaining the discrepancy.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question