
Sia

Question
Chemistry
Posted almost 2 years ago
(c) (S)-Amlodipine is used to treat high blood pressure and angina. The specific rotation of clinical grade -amlodipine is .
-amlodipine
The experimentally determined specific rotation of a batch prepared in the development laboratory using an alternative synthesis route is .
(i) Determine the optical purity and enantiomeric excess for this sample.
(ii) Use these values to calculate the percentage of the (+) and (-) enantiomer present in the laboratory sample. Show clearly how you reached your answers.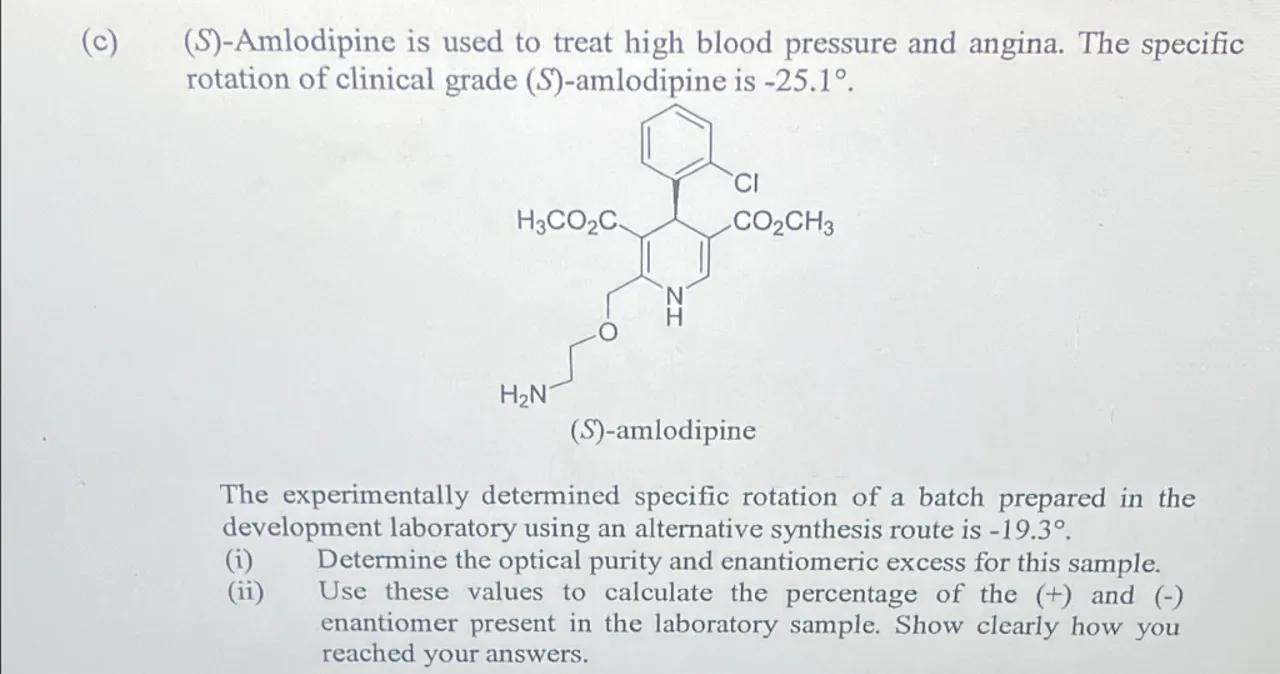

Answer from Sia
Posted almost 2 years ago
Solution
1
Calculate the observed specific rotation of the sample: The observed specific rotation of the laboratory sample of (S)-amlodipine is given as
2
Calculate the specific rotation of the pure enantiomer: The specific rotation of the pure (S)-amlodipine is given as
3
Determine the optical purity: Optical purity (also known as enantiomeric excess, ee) is calculated using the formula . Substituting the given values,
4
Calculate the enantiomeric excess: Performing the calculation from step 3,
5
Calculate the percentage of each enantiomer: The enantiomeric excess gives the excess of one enantiomer over the other. Since the sample is partially optically pure, we assume a 50% baseline for a racemic mixture. The percentage of the (S)-enantiomer is and the percentage of the (R)-enantiomer is
6
Determine the percentage of the (S)-enantiomer: Using the ee value from step 4, the percentage of the (S)-enantiomer is
7
Determine the percentage of the (R)-enantiomer: Similarly, the percentage of the (R)-enantiomer is
1 Answer
Optical purity (ee) is approximately 76.9%. The laboratory sample contains approximately 88.45% of the (S)-enantiomer and 11.55% of the (R)-enantiomer.
Key Concept
Optical purity and enantiomeric excess are measures of the composition of a chiral mixture in terms of its enantiomers.
Explanation
The optical purity is calculated by comparing the observed specific rotation of a sample to that of the pure enantiomer. The enantiomeric excess is used to determine the relative percentages of each enantiomer in the sample.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question