
Sia
consider the galvanic cell constructed with the following materials electrode, (NO
3)2, Fe electrode, . sketch a diagram of the galvanic cell identifying each material and labeling the cathode and anode determine the overall cell reaction and calculate cell c calculate delta and for the cell reaction at 25 degrees celsius . calculate Ecell at 25 degrees celcius when times 10 to the and [Fe ] 1.0 times 10 to the -4 th 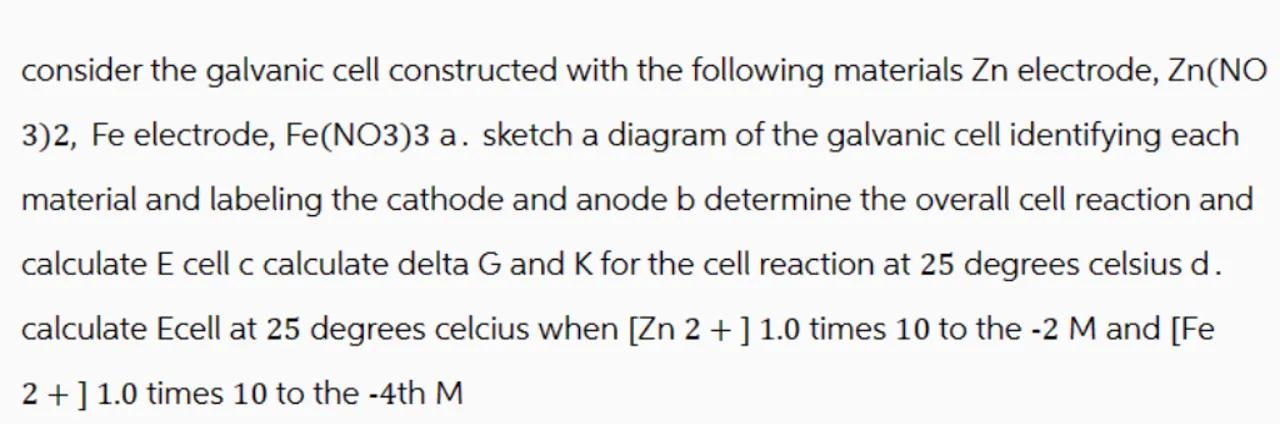

Answer from Sia
Posted almost 2 years ago
Solution
1
Sketch the diagram: A galvanic cell consists of two half-cells, each containing an electrode and an electrolyte. The Zn electrode is the anode where oxidation occurs, and the Fe electrode is the cathode where reduction occurs. The anode and cathode are connected by a salt bridge, and the external circuit connects the electrodes to allow for electron flow
2
Determine the overall cell reaction: The anode half-reaction is , and the cathode half-reaction is . The overall cell reaction is
3
Calculate $E_{\text{cell}}$: The standard electrode potentials () for the half-reactions are and . The standard cell potential is
4
Calculate $\Delta G$ and $K$: The change in free energy is calculated using the equation , where is the number of moles of electrons transferred (2 moles), is Faraday's constant (), and is the standard cell potential. The equilibrium constant is calculated using the equation , where is the gas constant (8.314\ \text{J/(mol·K)}) and is the temperature in Kelvin (298 K)
5
Calculate $E_{\text{cell}}$ at given concentrations: Use the Nernst equation , where is the reaction quotient. Given the concentrations,
1 Answer
Diagram not provided, but the description of the galvanic cell components and their arrangement is given.
2 Answer
The overall cell reaction is .
3 Answer
.
4 Answer
and can be calculated from .
5 Answer
at the given concentrations can be calculated using the Nernst equation.
Key Concept
Galvanic cells convert chemical energy into electrical energy through redox reactions, and the cell potential can be calculated using standard potentials and the Nernst equation.
Explanation
The galvanic cell operates on the principle of spontaneous redox reactions, with the anode experiencing oxidation and the cathode experiencing reduction. The standard cell potential gives the voltage under standard conditions, while the Nernst equation allows for the calculation of cell potential under non-standard conditions.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question
