
Sia

Question
Chemistry
Posted almost 2 years ago
d) Predict the structures of the product(s) and provide a mechanism for the following reaction. Use the solid/dash wedge convention to show three dimensions. Explain the stereochemical outcome.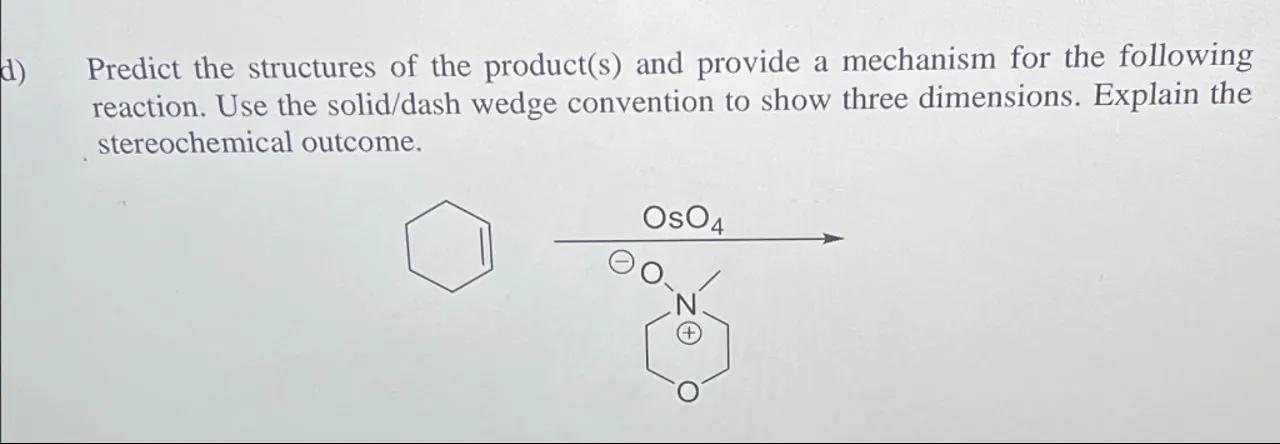

Answer from Sia
Posted almost 2 years ago
Solution
1
Identify the reaction: The reaction described is an osmium tetroxide-catalyzed dihydroxylation of cyclohexene
2
Predict the product structure: The product of this reaction will be a cis-diol, with two hydroxyl groups added to the former double bond of cyclohexene. The stereochemistry will be cis due to the syn addition of the osmium tetroxide reagent
3
Provide a mechanism: The mechanism involves the formation of a cyclic osmate ester intermediate, followed by a reductive workup that cleaves the osmium-oxygen bonds and replaces them with hydrogen-oxygen bonds
4
Explain the stereochemical outcome: The addition of the hydroxyl groups occurs on the same side of the double bond (syn addition), resulting in a cis-diol. This is because the osmium tetroxide adds to the alkene in a concerted manner, forming a cyclic intermediate that preserves the relative positions of the substituents
Answer
The product is a cis-diol derived from cyclohexene, with both hydroxyl groups on the same side of the former double bond. The mechanism involves the formation of a cyclic osmate ester intermediate with syn addition of hydroxyl groups.
Key Concept
Dihydroxylation of alkenes with OsO4
Explanation
OsO4 adds to alkenes like cyclohexene to form a cyclic osmate ester, which upon reductive workup yields a cis-diol with retention of stereochemistry due to syn addition.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question