
Sia

Question
Chemistry
Posted almost 2 years ago
(e) For the structures below, state whether the hydrogens marked and are homotopic, enantiotopic or diastereotopic and give a reason for your answer.
(i)
(ii)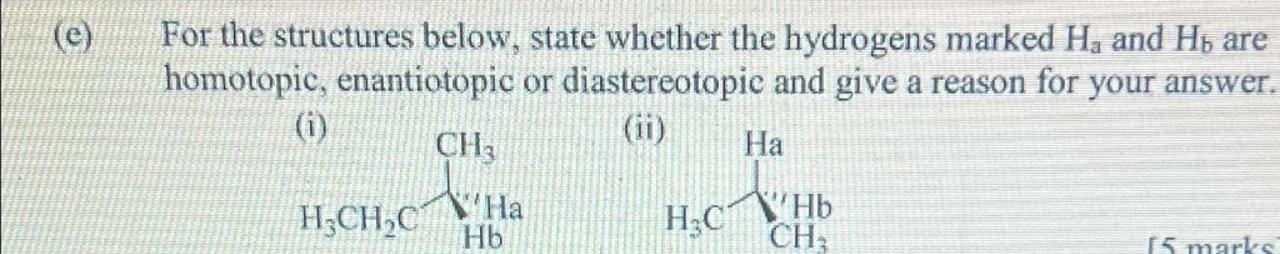

Answer from Sia
Posted almost 2 years ago
Solution
1
Determine the symmetry elements of the molecules: To classify the hydrogens as homotopic, enantiotopic, or diastereotopic, we need to analyze the symmetry of the molecule and the environment around the hydrogens
2
Analyze molecule (i): For structure (i), we have a central carbon atom bonded to a methyl group (), an ethyl group (), and a hydrogen atom (H). The two hydrogens and are attached to the central carbon. Since replacing either or with a deuterium atom would generate two different compounds that are not mirror images, and are diastereotopic
3
Analyze molecule (ii): For structure (ii), we have a central carbon atom bonded to two methyl groups and a hydrogen atom (H). The two hydrogens and are attached to the central carbon. Replacing or with a deuterium atom would generate two compounds that are mirror images of each other, which means and are enantiotopic
1 Answer
For structure (i), the hydrogens marked and are diastereotopic. For structure (ii), the hydrogens marked and are enantiotopic.
Key Concept
Homotopic hydrogens are identical and interchangeable, enantiotopic hydrogens are mirror images and not superimposable, and diastereotopic hydrogens are neither identical nor mirror images.
Explanation
In structure (i), the hydrogens are diastereotopic because replacing them with deuterium results in different, non-mirror image compounds. In structure (ii), the hydrogens are enantiotopic because replacing them with deuterium results in mirror image compounds.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question