
Sia
i want to know this reaction mechanism by eletron pushing
hot - electron
SPR - activation silver atom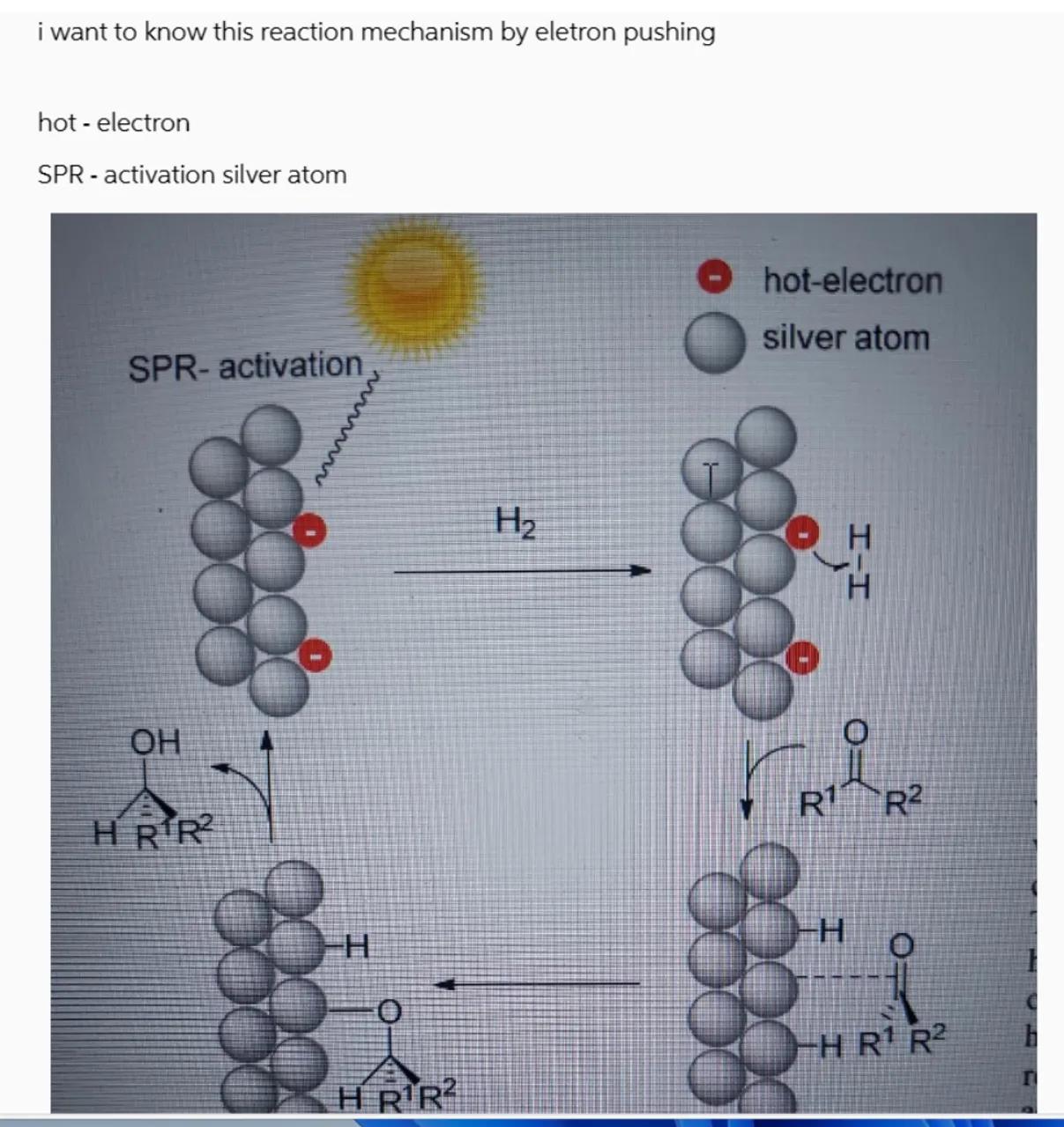

Answer from Sia
Posted almost 2 years ago
Solution
1
Identify the reaction mechanism: The reaction mechanism involves surface plasmon resonance (SPR) activation of a silver atom cluster which facilitates the transfer of a "hot electron" to an alcohol molecule
2
Electron pushing in the mechanism: The "hot electron" from the silver cluster is transferred to the alcohol molecule, initiating a series of bond rearrangements. This results in the removal of a hydrogen atom from the alcohol, which pairs with another hydrogen atom to form . The alcohol is then bound to the silver cluster through the oxygen atom
3
Formation of products: The removal of the hydrogen atom from the alcohol can lead to the formation of an aldehyde if the hydrogen is removed from the oxygen, or a different product if the hydrogen is removed from the carbon atom (R1)
Answer
The reaction mechanism involves SPR-activation of a silver atom cluster, electron transfer to an alcohol molecule, and subsequent bond rearrangements leading to the formation of an aldehyde or another product depending on the site of hydrogen atom removal.
Key Concept
Electron transfer in reaction mechanisms
Explanation
In this reaction, a "hot electron" is transferred to an alcohol molecule, causing a hydrogen atom to be removed and bond rearrangements to occur, leading to product formation.
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question
