
Sia
hydrarine
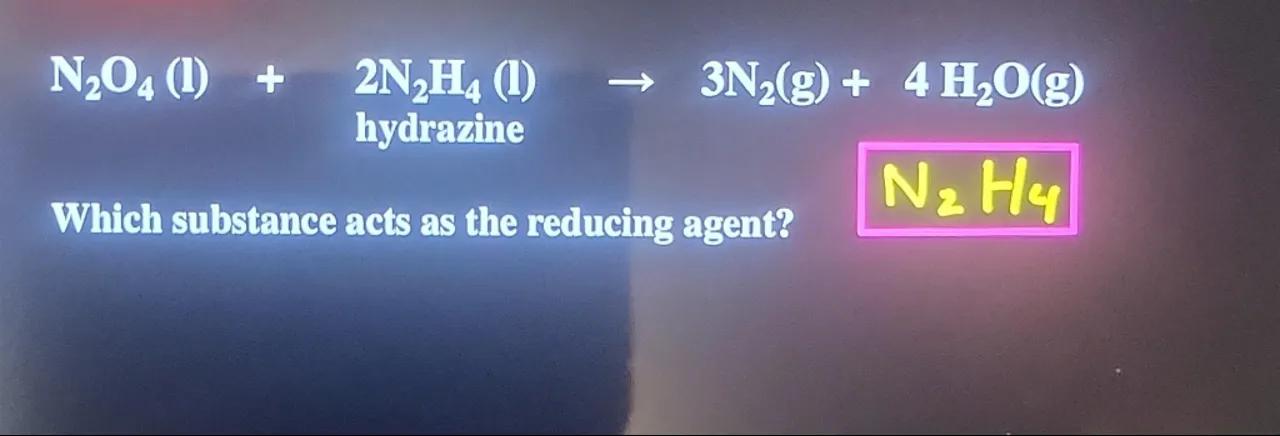
Which substance acts as the reducing agent?

Answer from Sia
Posted almost 2 years ago
D
Key Concept
Reducing Agent
Explanation
In the given reaction, (hydrazine) is the reducing agent because it donates electrons to , resulting in its reduction to , while itself gets oxidized to .
Not the question you are looking for? Ask here!
Enter question by text
Enter question by image
Unlock Smarter Learning with AskSia Super!
Join Super, our all-in-one AI solution that can greatly improve your learning efficiency.
30% higher accuracy than GPT-4o
Entire learning journey support
The most student-friendly features
Study Other Question
